N-(4-Chlorophenyl)acetamide - 2,4,6-trinitrophenol (1:1) | CAS No. 114069-77-1
Basic Information
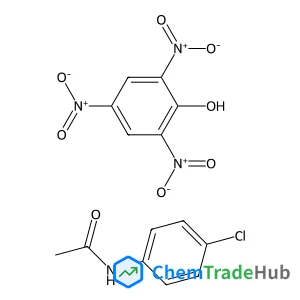
CAS Number
114069-77-1
Molecular Formula
C14H11ClN4O8
Molecular Weight
398.71 g/mol
Quick Actions
Basic Physical Properties
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationSynonyms & References
English
- N-(4-chlorophenyl)acetamide,2,4,6-trinitrophenol
- Acetamide, N-(4-chlorophenyl)-, compd. with 2,4,6-trinitrophenol (1:1)
- N-(4-Chlorophenyl)acetamide--2,4,6-trinitrophenol (1/1)
- N-(4-Chlorophenyl)acetamide;2,4,6-trinitrophenol
CAS Number
114069-77-1
Recommended Suppliers
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Yongqing County Chengja Chemical Co., Ltd. China - Yongqing County Chengja Chemical Co., Ltd. |
|||||
 United States - AJAY NORTH AMERICA, L.L.C. United States - AJAY NORTH AMERICA, L.L.C. |
|||||
 China - Shanghai Kain Biopharm Technology Co., Ltd. China - Shanghai Kain Biopharm Technology Co., Ltd. |
|||||
 China - Dezhou Jiatai Chemical Technology Co., Ltd. China - Dezhou Jiatai Chemical Technology Co., Ltd. |
|||||
 El Salvador - Comercio y Representaciones SA de CV (Coresa de CV) El Salvador - Comercio y Representaciones SA de CV (Coresa de CV) |
|||||
 China - Baoji Baoyi Titanium Nickel Manufacturing Co., Ltd. China - Baoji Baoyi Titanium Nickel Manufacturing Co., Ltd. |
|||||
 Germany - QUANCOM Informationssysteme GmbH Germany - QUANCOM Informationssysteme GmbH |
|||||
 India - Indogulf Company India - Indogulf Company |
Related Compounds
Related Articles
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloaddition
Renzeng Chen, Danbo Wang, Wenbo Hao, Feng Shao, Yingjie Zhao
DOI: 10.1039/D1CC02179F
A hollow neuronal carbon skeleton with ultrahigh pyridinic N content as a self-supporting potassium-ion battery anode
Yongwen Sun, Ya Zhang, Zheng Xing, Denghu Wei, Quanchao Zhuang
DOI: 10.1039/C9SE00889F
Effective utilisation of waste cooking oil in a single-cylinder diesel engine using alumina nanoparticles
Sumit Roy, Pranay Kumar Parsi, R. Sreeram Kotha, Sanmitra Barman, Kalluri Vinayak, Mili Mitra Roy, Rahul Banerjee
DOI: 10.1039/C9SE00393B
Carbon-based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels
Mufeedah Muringa Kandy
DOI: 10.1039/C9SE00827F
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
Highly efficient and durable III–V semiconductor-catalyst photocathodes via a transparent protection layer
Shinjae Hwang, James L. Young, Rachel Mow, Mengjun Li, Hongbin Yang, Philip E. Batson, Martha Greenblatt, Myles A. Steiner, Daniel Friedman, Todd G. Deutsch, Eric Garfunkel
DOI: 10.1039/C9SE01264H
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H