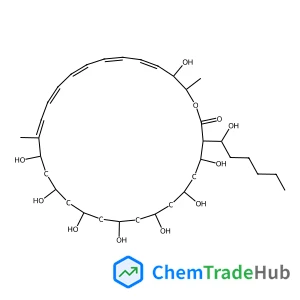
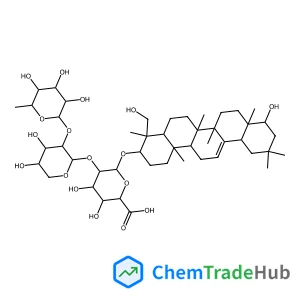
2,6,6-Trimethyl-4-methylene-1,3,2-oxathiaphosphinane 2-sulfide | CAS No. 114067-83-3
Basic Information
CAS Number
114067-83-3
Molecular Formula
C7H13OPS2
Molecular Weight
208.29 g/mol
Quick Actions
Basic Physical Properties
Classification & Uses
Chemical Classification
Safety Information
View Safety InformationSynonyms & References
English
- 2,6,6-trimethyl-4-methylidene-2-sulfanylidene-1,3,2λ<sup>5</sup>-oxathiaphosphinane
- 1,3,2-Oxathiaphosphorinane, 2,6,6-trimethyl-4-methylene-, 2-sulfide
- 2,6,6-Trimethyl-4-methylidene-1,3,2lambda~5~-oxathiaphosphinane-2-thione
CAS Number
114067-83-3
Recommended Suppliers
| Supplier Name | Member Level | Authentication Status | Main Category | MOQ | Action |
|---|---|---|---|---|---|
 China - Shouguang Shendá Chemical Industry Co., Ltd. China - Shouguang Shendá Chemical Industry Co., Ltd. |
|||||
 United Kingdom - Elite Thermal Systems Ltd United Kingdom - Elite Thermal Systems Ltd |
|||||
 Belgium - EVAL Europe N.V. Belgium - EVAL Europe N.V. |
|||||
 China - Shenzhen Zhongshenghe Real Estate Co., Ltd. China - Shenzhen Zhongshenghe Real Estate Co., Ltd. |
|||||
 China - Liyang Chermlawn Chemical Co., Ltd. China - Liyang Chermlawn Chemical Co., Ltd. |
|||||
 China - Nanjing Kunchen Chemical Industry Co., Ltd. China - Nanjing Kunchen Chemical Industry Co., Ltd. |
|||||
 Germany - NMI Naturwissenschaftliches und Medizinisches Institut an der Universität Tübingen Germany - NMI Naturwissenschaftliches und Medizinisches Institut an der Universität Tübingen |
|||||
 Italy - Variati & Co. S.p.a. Italy - Variati & Co. S.p.a. |
Related Compounds
Recommended Journals

Australian Journal of Chemistry

Journal of the Chinese Chemical Society

Canadian Metallurgical Quarterly

Biopolymers

Advances in Colloid and Interface Science

Chemistry of Heterocyclic Compounds

Bulletin of the Chemical Society of Japan

Cement and Concrete Research

Journal of the American Chemical Society

Accounts of Chemical Research
Related Articles
Metal–organic frameworks: preparation and applications in highly efficient heterogeneous photocatalysis
Van-Huy Nguyen, Shi-Rong Zhou, Shu-Yu Hsu, Jia-Xuan Tan
DOI: 10.1039/C9SE00972H
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
From Douglas fir to renewable H2-enriched syngas via ex situ catalytic pyrolysis over metal nanoparticles–nanocellulose derived carbon catalysts
Hanwu Lei, Chenxi Wang, Moriko Qian, Elmar Villota, Wendy Mateo
DOI: 10.1039/C9SE00860H
Direct arylation polycondensation towards water/alcohol-soluble conjugated polymers as the electron transporting layers for organic solar cells
Qingwu Yin, Zhenfeng Wang, Boming Xie, Fei Huang, Yong Cao
DOI: 10.1039/D1CC01128F
The limits to biocatalysis: pushing the envelope
Roger A. Sheldon, Dean Brady
DOI: 10.1039/C8CC02463D
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Photoactivatable fluorophores for durable labelling of individual cells
Hiroki Kashima, Mako Kamiya, Shotaro Nakano, Masayuki Miura
DOI: 10.1039/D1CC01488A
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
CaMoO4 nanosheet arrays for efficient and durable water oxidation electrocatalysis under alkaline conditions
Ying Gou, Qin Liu, Xifeng Shi, Abdullah M. Asiri, Jianming Hu, Xuping Sun
DOI: 10.1039/C8CC02092B
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J

![59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione) 59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione)](/structs/591/59156-70-6-0f4b.webp)