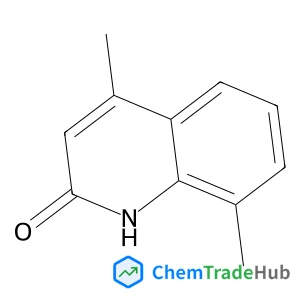
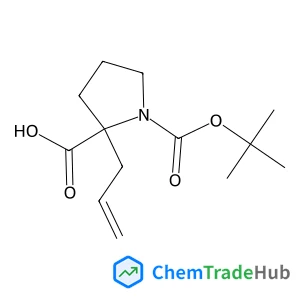
Enantioselective [2 + 2] cycloaddition of 1,2-dihydroquinolines with 3-olefinic oxindoles via Brønsted acid catalysis
文献信息
Biao Wang, Xiao Yan, Han Zhong, Qin Ouyang, Xu Tian
Mutually complementary regiodivergent Brønsted acid-catalyzed atom-economical [2 + 2] cycloaddition and ene reactions of 1,2-dihydroquinolines with 3-olefinic oxindoles are reported. In the presence of a chiral phosphoramide catalyst, the [2 + 2] cycloaddition affords products with four contiguous stereocenters in good to excellent yields (up to 95%) and with high stereoselectivities (up to >99% ee, >20 : 1 dr). Conversely, with a stronger Brønsted acid, the trifluoromethanesulfonic acid catalyst leads to ene reaction products in high yields (up to 77%). Furthermore, the mechanisms of the reactions are discussed based on control experiments and DFT calculations.
相关文献
IF 6.222
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structureIF 6.222
Back coverIF 6.222
Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer's disease brain tissueIF 6.222
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosisIF 6.222
Direct arylation polycondensation towards water/alcohol-soluble conjugated polymers as the electron transporting layers for organic solar cellsIF 6.222
Strong circularly polarized luminescence of an octahedral chromium(iii) complexIF 6.222
MnO/C cubo-polyhedrons derived from α-MnO2@ZIF-8 as anode materials for high-performance lithium-ion batteriesIF 6.367
Contents listIF 6.843
Catalogue of self-targeting nano-medical inventions to accelerate clinical trialsIF 6.843
来源期刊
Organic Chemistry Frontiers

Organic Chemistry Frontiers publishes high-quality research from across organic chemistry. Emphases are placed on studies that make significant contributions to the field of organic chemistry by reporting either new or significantly improved protocols or methodologies. Topics include, but are not limited to the following: Organic synthesis Development of synthetic methodologies Catalysis Natural products Functional organic materials Supramolecular and macromolecular chemistry Physical and computational organic chemistry
推荐供应商
 佛山市安你心香精香料有限公司
佛山市安你心香精香料有限公司 URACA GmbH&Co.KG
URACA GmbH&Co.KG 陕西晨曦之光化学科技有限公司
陕西晨曦之光化学科技有限公司 Mci-miritz柑橘配料有限公司
Mci-miritz柑橘配料有限公司 天津立悦合信科技有限公司
天津立悦合信科技有限公司 苏州升井环保设备有限公司
苏州升井环保设备有限公司 南京延乔科技有限公司
南京延乔科技有限公司 武汉武大弘元股份有限公司
武汉武大弘元股份有限公司 马希宁·梅切尔
马希宁·梅切尔 寿光市申达化学工业有限公司
寿光市申达化学工业有限公司










![503070-57-3 - 2-[2-(6-溴己氧基)乙氧基甲基]-1,3-二氯苯 503070-57-3 - 2-[2-(6-溴己氧基)乙氧基甲基]-1,3-二氯苯](/structs/503/503070-57-3-bc25.webp)