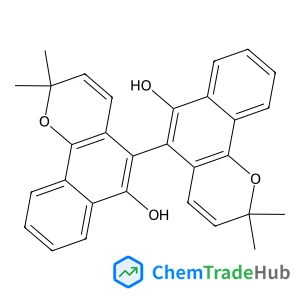
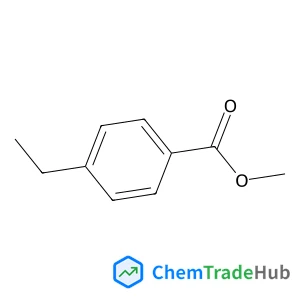
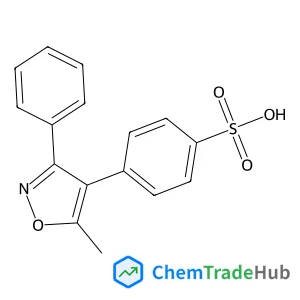
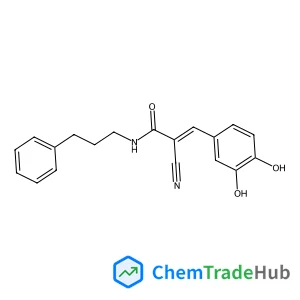
Understanding the remarkable difference in liquid crystal behaviour between secondary and tertiary amides: the synthesis and characterisation of new benzanilide-based liquid crystal dimers
文献信息
Grant J. Strachan, William T. A. Harrison, John M. D. Storey, Corrie T. Imrie
A number of liquid crystal dimers have been synthesised and characterised containing secondary or tertiary (N-methyl) benzanilide-based mesogenic groups. The secondary amides all form nematic phases, and we present the first example of an amide to show the twist-bend nematic (NTB) phase. Only two of the corresponding N-methylated dimers formed a nematic phase and with greatly reduced nematic–isotropic transition temperatures. Characterisation using 2D ROESY NMR experiments, DFT geometry optimisation and X-ray diffraction reveal that there is a change in the preferred conformation of the benzanilide core on methylation, from Z to E. The rotational barrier around the N–C(O) bond has been measured using variable temperature 1H NMR spectroscopy. This dramatic change in shape accounts for the remarkable difference in liquid crystalline behaviour between these secondary and tertiary amide-based materials.
相关文献
IF 6.222
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen sourceIF 6.367
An elemental S/P photocatalyst for hydrogen evolution from water under visible to near-infrared light irradiationIF 6.222
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a reviewIF 6.222
Metal–organic frameworks: preparation and applications in highly efficient heterogeneous photocatalysisIF 6.367
Heterogeneous toroidal spiral particles for islet encapsulationIF 6.843
Recent developments in carbon nitride based films for photoelectrochemical water splittingIF 6.367
Life cycle assessment of power-to-gas with biogas as the carbon sourceIF 6.367
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosityIF 6.367
Permselective ion electrosorption of subnanometer pores at high molar strength enables capacitive deionization of saline waterIF 6.367
来源期刊
Physical Chemistry Chemical Physics

Physical Chemistry Chemical Physics (PCCP) is an international journal co-owned by 19 physical chemistry and physics societies from around the world. This journal publishes original, cutting-edge research in physical chemistry, chemical physics and biophysical chemistry. To be suitable for publication in PCCP, articles must include significant innovation and/or insight into physical chemistry; this is the most important criterion that reviewers and Editors will judge against when evaluating submissions. The journal has a broad scope and welcomes contributions spanning experiment, theory, computation and data science. Topical coverage includes spectroscopy, dynamics, kinetics, statistical mechanics, thermodynamics, electrochemistry, catalysis, surface science, quantum mechanics, quantum computing and machine learning. Interdisciplinary research areas such as polymers and soft matter, materials, nanoscience, energy, surfaces/interfaces, and biophysical chemistry are welcomed if they demonstrate significant innovation and/or insight into physical chemistry. Joined experimental/theoretical studies are particularly appreciated when complementary and based on up-to-date approaches.
推荐供应商
 江西拓昊福生物科技有限公司
江西拓昊福生物科技有限公司 ApiniLabs股份公司
ApiniLabs股份公司 Embion技术有限公司
Embion技术有限公司 FWA Friedrich Werntges Apparatebau GmbH
FWA Friedrich Werntges Apparatebau GmbH 天津拓华泛恩化工有限公司
天津拓华泛恩化工有限公司 深圳市优品生物科技有限公司黄石分公司
深圳市优品生物科技有限公司黄石分公司 DECKMA HAMBURG GmbH
DECKMA HAMBURG GmbH 埃德尔斯塔尔服务公司
埃德尔斯塔尔服务公司 QUMA Elektronik & Analytik GmbH
QUMA Elektronik & Analytik GmbH 山东亿淳化学有限公司
山东亿淳化学有限公司