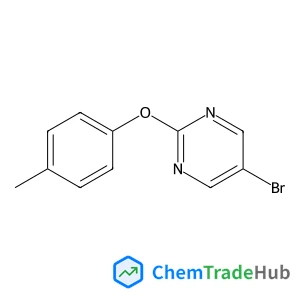
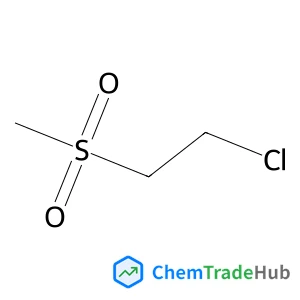
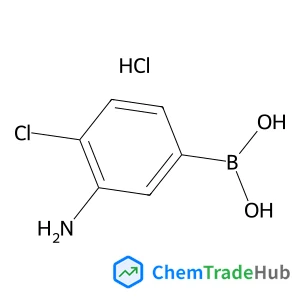
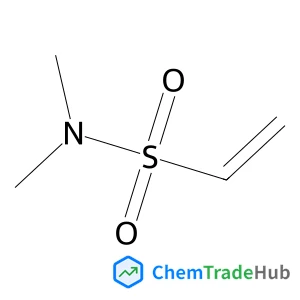
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
文献信息
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
The tandem synthesis of benzimidazole and other azoles can be achived by the N-formylation of ortho-substituted anilines followed by a cyclization reaction. However, CO2-based N-formylations with hydrosilane reducing agents are base catalyzed whereas the cyclization reaction is acid catalyzed. The mismatch in catalytic conditions means that only one of the steps can be catalyzed in a single pot reaction. While the N-formylation reaction is frequently the target of catalyst development, the cyclization reaction requires comparably much harsher reaction conditions. Identification of these difficulties lead us to the development of a one-pot, two-step synthesis of benzimidazole under mild reaction conditions employing acid catalysts.
相关文献
IF 6.367
Outstanding Reviewers for ChemComm in 2020IF 6.222
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosityIF 6.367
An environmentally friendly natural polymer as a universal interfacial modifier for fullerene and non-fullerene polymer solar cellsIF 6.367
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping systemIF 6.367
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursorIF 6.222
Life cycle assessment of plasma-assisted ethylene production from rich-in-methane gas streamsIF 6.367
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular deviceIF 6.367
Life cycle assessment of power-to-gas with biogas as the carbon sourceIF 6.367
Pulsed laser rusted stainless steel: a robust electrode material applied for energy storage and generation applicationsIF 6.367
来源期刊
Chemical Communications

ChemComm publishes urgent research which is of outstanding significance and interest to experts in the field, while also appealing to the journal’s broad chemistry readership. Our communication format is ideally suited to short, urgent studies that are of such importance that they require accelerated publication. Our scope covers all topics in chemistry, and research at the interface of chemistry and other disciplines (such as materials science, nanoscience, physics, engineering and biology) where there is a significant novelty in the chemistry aspects. Major topic areas covered include: Analytical Chemistry Catalysis Chemical Biology and medicinal chemistry Computational Chemistry and Machine Learning Energy and sustainable chemistry Environmental Chemistry Green Chemistry Inorganic Chemistry Materials Chemistry Nanoscience Organic Chemistry Physical Chemistry Polymer Chemistry Supramolecular Chemistry
推荐供应商
 Interstuhl Büromöbel GmbH & Co. KG
Interstuhl Büromöbel GmbH & Co. KG ARGUS环境生物技术有限公司
ARGUS环境生物技术有限公司 DYMATIC化学公司
DYMATIC化学公司 深圳市中盛和实业有限公司
深圳市中盛和实业有限公司 布赫工业公司
布赫工业公司 Alquim Especialidades Químicas,SA de C.V.
Alquim Especialidades Químicas,SA de C.V. DaXem有限公司
DaXem有限公司 德国宾馆有限公司
德国宾馆有限公司 江西拓昊福生物科技有限公司
江西拓昊福生物科技有限公司 生物链公司
生物链公司