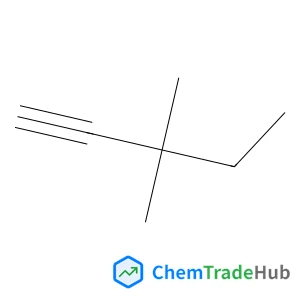
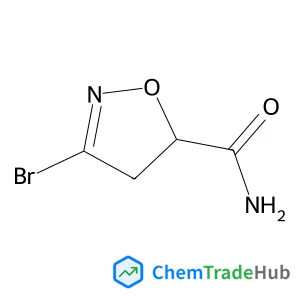
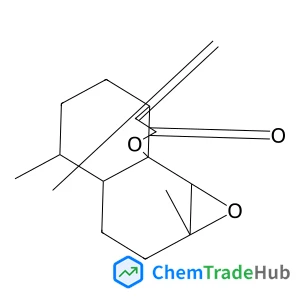
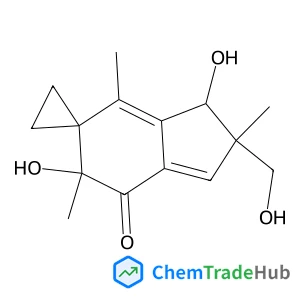
Dess–Martin periodinane oxidative rearrangement for preparation of α-keto thioesters
文献信息
Randy Sanichar, Ciaran Carroll, Ryan Kimmis, Bela Reiz, John C. Vederas
A Dess–Martin Periodinane (DMP) mediated oxidative rearrangement reaction was uncovered. The reaction proceeds via oxidation of a β-hydroxy thioester to a β-keto thioester, followed by an α-hydroxylation and then further oxidation to form a vicinal thioester tricarbonyl. This product then rearranges, extruding CO2, to form an α-keto product. The mechanism of the rearrangement was elucidated using 13C labelling and analysis of the intermediates as well as the products of the reaction. This efficient process allows for easy preparation of α-keto thioesters which are potential intermediates in the synthesis of pharmaceutically important heterocyclic scaffolds such as quinoxalinones.
相关文献
IF 6.367
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complexIF 6.222
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structureIF 6.222
Redox responsive Pluronic micelle mediated delivery of functional siRNA: a modular nano-assembly for targeted deliveryIF 6.843
Outstanding Reviewers for ChemComm in 2020IF 6.222
An aminophosphonate ester ligand-containing platinum(ii) complex induces potent immunogenic cell death in vitro and elicits effective anti-tumour immune responses in vivoIF 6.222
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen sourceIF 6.367
Milk exosomes with enhanced mucus penetrability for oral delivery of siRNAIF 6.843
Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA deliveryIF 6.843
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring systemIF 6.367
来源期刊
Organic & Biomolecular Chemistry

Organic & Biomolecular Chemistry (OBC) publishes original and high impact research and reviews in organic chemistry. We welcome research that shows new or significantly improved protocols or methodologies in total synthesis, synthetic methodology or physical and theoretical organic chemistry as well as research that shows a significant advance in the organic chemistry or molecular design aspects of chemical biology, catalysis, supramolecular and macromolecular chemistry, theoretical chemistry, mechanism-oriented physical organic chemistry, medicinal chemistry or natural products. Articles published in the journal should report new work which makes a highly-significant impact in the field. Routine and incremental work is generally not suitable for publication in the journal. More details about key areas of our scope are below. In all cases authors should include in their article clear rationale for why their research has been carried out.
推荐供应商
 DESMI有限公司
DESMI有限公司 IMTEX控制有限公司
IMTEX控制有限公司 昆山力电精密机械有限公司
昆山力电精密机械有限公司 Arch Química,S.A. de C.V.
Arch Química,S.A. de C.V. Ludwig Melosch Vertriebs-GmbH & Co.
Ludwig Melosch Vertriebs-GmbH & Co. 南京定达医药科技有限公司
南京定达医药科技有限公司 襄樊诺尔化工有限公司
襄樊诺尔化工有限公司 CEPSA
CEPSA 霍布雷仪器公司
霍布雷仪器公司 雷特克电子规范技术有限公司
雷特克电子规范技术有限公司