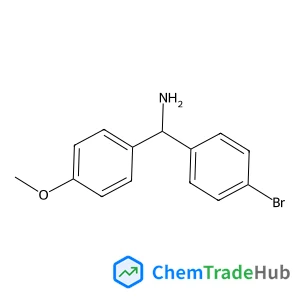
1-(4-Bromophenyl)-1-(4-methoxyphenyl)methanamine(CAS号:886362-84-1)
1-(4-溴苯基)-1-(4-甲氧基苯基)甲胺
基本信息
CAS号
886362-84-1
分子式
C14H14BrNO
分子量
292.18 g/mol
Quick Actions
基本物理性质
物理状态
White to Yellow Solid
沸点
408.4°C at 760 mmHg
密度
1.368
闪点
200.8°C
折射率
1.604
安全信息
查看安全信息危险说明
H302;H315;H319;H335
同义词与参考文献
英文
- FT-0740468
- AS-38548
- GSXAISCLDOQZOQ-UHFFFAOYSA-N
- AKOS022323541
- 1-(4-Bromophenyl)-1-(4-methoxyphenyl)methanamine
- (4-bromophenyl)-(4-methoxyphenyl)methanamine
- (4-bromophenyl)(4-methoxyphenyl)methanamine
- SCHEMBL14809243
- MFCD04115330
- CS-0172577
- A12978
- 1-(4-Bromophenyl)-1-(4-methoxyphenyl)methylamine
- 886362-84-1
- AKOS000169214
- DTXSID50640718
- c-(4-bromo-phenyl)-c-(4-methoxy-phenyl)-methylamine
中文
- 1-(4-溴苯基)-1-(4-甲氧基苯基)甲胺
MDL_Number
MFCD04115330
CAS号
886362-84-1
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海颖心实验室设备有限公司 中国 - 上海颖心实验室设备有限公司 |
|||||
 中国 - 上海捷世凯生物科技有限公司 中国 - 上海捷世凯生物科技有限公司 |
|||||
 中国 - 上海源叶生物科技有限公司 中国 - 上海源叶生物科技有限公司 |
|||||
 中国 - 无锡嘉屹化工有限公司 中国 - 无锡嘉屹化工有限公司 |
|||||
 瑞士 - Emile Egger&Cie SA 瑞士 - Emile Egger&Cie SA |
|||||
 德国 - 丁克尔伯格分析有限公司 德国 - 丁克尔伯格分析有限公司 |
|||||
 中国 - 偃师市东园化工有限公司 中国 - 偃师市东园化工有限公司 |
|||||
 德国 - 克莱恩纺织品 德国 - 克莱恩纺织品 |
期刊推荐

Journal of the Chinese Chemical Society

Bulletin of the Chemical Society of Japan

Journal of the American Chemical Society

Chemistry of Natural Compounds

Analyst

Advances in Colloid and Interface Science

Chemistry of Heterocyclic Compounds

Anti-Corrosion Methods and Materials

Cement and Concrete Research

Biopolymers
相关文献
Direct arylation polycondensation towards water/alcohol-soluble conjugated polymers as the electron transporting layers for organic solar cells
Qingwu Yin, Zhenfeng Wang, Boming Xie, Fei Huang, Yong Cao
DOI: 10.1039/D1CC01128F
Transition metal chemistry in synthetically viable alkaline earth complexes M(Cp)3− (M = Ca, Sr, Ba)
Bin Huo, Rui Sun, Bo Jin, Lingfei Hu, Jian-Hong Bian, Xiao-Ling Guan, Caixia Yuan, Gang Lu, Yan-Bo Wu
DOI: 10.1039/D1CC01753E
A hollow neuronal carbon skeleton with ultrahigh pyridinic N content as a self-supporting potassium-ion battery anode
Yongwen Sun, Ya Zhang, Zheng Xing, Denghu Wei, Quanchao Zhuang
DOI: 10.1039/C9SE00889F
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
Cu2ZnSnS4 nanocrystals for microwave thermal and microwave dynamic combination tumor therapy
Taya Tang, Xiaomu Xu, Zhiwen Wang, Jijing Tian, Yue Yang, Caizhang Ou, Huihui Bao, Tianlong Liu
DOI: 10.1039/C9CC07762F
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H
Electrocatalytic cleavage of lignin model dimers using ruthenium supported on activated carbon cloth
Mahlet Garedew, Daniel Young-Farhat, Souful Bhatia, Pengchao Hao, James E. Jackson
DOI: 10.1039/C9SE00912D
Engineering nanoporous organic frameworks to stabilize naked Au clusters: a charge modulation approach
Chengcheng Tian, Xiang Zhu, Huize Wang, Hai Wang, Carter W. Abney, Ning Zhang
DOI: 10.1039/C8CC02966K
Synthesis of aviation fuel from bio-derived isophorone
Courtney Ford Ryan, Cameron M. Moore, Juan H. Leal, Troy A. Semelsberger, Jenny K. Banh, Junqing Zhu, Charles S. McEnally, Lisa D. Pfefferle, Andrew D. Sutton
DOI: 10.1039/C9SE01014A

![25553-77-9 - 2-[2-(哌嗪-1-基)-乙基]-1,3-二氧杂烷 25553-77-9 - 2-[2-(哌嗪-1-基)-乙基]-1,3-二氧杂烷](/structs/255/25553-77-9-5274.webp)