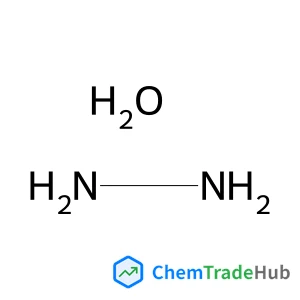
Hydrazine hydrate (1:1)(CAS号:7803-57-8)
水合肼
基本信息
CAS号
7803-57-8
分子式
H6N2O
分子量
50.06 g/mol
Quick Actions
基本物理性质
熔点
-51.5ºC
沸点
120.1ºC
密度
1.032
水溶性
Soluble in chloroform, DMSO, methanol. Slightly soluble in water.
闪点
75ºC
折射率
1.4285-1.4315
蒸气压
5 mm Hg ( 25 °C)
安全信息
查看安全信息危险说明
H226,H301,H311,H314,H317,H331,H350,H410
危险类别
8
稳定性
Stability Incompatible with a wide variety of materials, including oxidizing agents, heavy metal oxides, dehydrating agents, alkali metals, rust, silver salts. Combustible. Contact with many materials may cause fire or explosive decomposition. May react explosively with a variety of materials, including dehydrating agents, heavy metal ox
爆炸极限
>3.4-99%(V)
同义词与参考文献
英文
- J-000638
- hydraziniumhydroxide
- hydrazine-hydrate
- NH2-NH2-H2O
- Q3143689
- Hydrazine monohydrate, SAJ first grade, >=98.0%
- hydrazin, hydrate
- H2NNH2.H2O
- CCRIS 7739
- NS00113412
- STR00022
- A839316
- CHEBI:35511
- diazane hydrate
- 10217-52-4
- hydrazine mono-hydrate
- NH2NH2-H2O
- IKDUDTNKRLTJSI-UHFFFAOYSA-N
- mono hydrazine hydrate
- hydrazine;hydrate
- Hydrazinehydrate 
- HYDRAZINE, MONOHYDRATE
- HYDRAZINE MONOHYDRATE [MI]
- UNII-KYD297831P
- AKOS015855338
- 7803-57-8
- Idrazina idrata [Italian]
- hydrazine hydrat
- Hydrazinium hydroxide
- Hydrazine hydrate
- NH2-NH2.H2O
- Hydrazine, hydrate (1:1)
- DTXSID9037240
- hydrazine.hydrate
- Hydrazine monohydrate pound>>Hydrazinium hydroxide pound>>Hydrazinehydrate
- Hydrazine, hydrate
- NH2NH2 H2O
- Idrazina idrata
- N2 H4 H2 O
- hydrazine mono hydrate
- hydrazin hydrate
- hyrazine hydrate
- MFCD00149931
- hyrazine monohydrate
- H2NNH2 H2O
- H2NNH2-H2O
- DTXSID901014833
- NH2NH2 hydrate
- Hydrazine hydrate (1:1)
- Hydrazine monohydrate, SAJ special grade, >=98.0%
- hydrate hydrazine
- J-610002
- hydrazine-monohydrate
- KYD297831P
- hydrazine H2O
- NH2NH2.H2O
- H2N-NH2.H2O
- Hydrazine monohydrate, N2H4 64-65 %, reagent grade, 98%
- Hydrazine hydroxide
- Hydrazine hydrate, reagent grade, N2H4 50-60 %
- BCP32127
- Hydrazine monohydrate
- NH2NH2 water
- hydrazine--water (1/1)
- hydrazine. hydrate
- Hydrazine hydrate solution 60 (N2H4.H2O)
- Diamid hydrate
- DiamideHydrate
- DiamineHydrate
- hydrazinehydroxide
- idrazinaidrata
- DiaMide hydrate
- Heptadecane
中文
- 水合联氨
- 含水肼
- 含水联氨
- 一水合肼
- 单水合肼
- Hydrazine Monohydrate 肼一水合物
- 肼,一水合物
- 吗啉
- 氢氧化肼
- 水合肼
- 水合肼 一水合物
- 水合肼(水合联氨)
- 肼,水合
- 十七烷
- 水合肼,肼 一水合物
- 水合肼,肼 一水合物,Hydrazine monohydrate
- 水合肼,一水
- 水合联氨,水合肼
MDL_Number
MFCD00149931
CAS号
7803-57-8
Customs_Code
2825109000
Merck_Index
14,4771
推荐供应商
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 广州旭帆贸易有限公司 中国 - 广州旭帆贸易有限公司 |
|||||
 美国 - 格罗顿生物系统公司 美国 - 格罗顿生物系统公司 |
|||||
 中国 - 南京坤成化工有限公司 中国 - 南京坤成化工有限公司 |
|||||
 奥地利 - Waagen Scheffknecht有限公司 奥地利 - Waagen Scheffknecht有限公司 |
|||||
 德国 - 克劳迪乌斯·彼得斯集团 德国 - 克劳迪乌斯·彼得斯集团 |
|||||
 德国 - 卡尔蔡司自动检测有限公司 德国 - 卡尔蔡司自动检测有限公司 |
|||||
 德国 - 化学检验有限公司 德国 - 化学检验有限公司 |
|||||
 中国 - 云南氟业 中国 - 云南氟业 |
相关文献
A new neodymium–phosphine compound for supercapacitors with long-term cycling stability
Xiaoyu Li, Huimin Chen, Chenyu Yang, Yafeng Li
DOI: 10.1039/D1CC00650A
Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cells
R. Vani, S. Ramaprabhu, Prathap Haridoss
DOI: 10.1039/C9SE01031A
A hollow neuronal carbon skeleton with ultrahigh pyridinic N content as a self-supporting potassium-ion battery anode
Yongwen Sun, Ya Zhang, Zheng Xing, Denghu Wei, Quanchao Zhuang
DOI: 10.1039/C9SE00889F
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalysts
Minling Fang, Zhiping Zheng, Jiayu Chen, Qian Chen, Deyu Liu, Binbin Xu, Jianyang Wu, Qin Kuang
DOI: 10.1039/C9SE00678H
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F
Catalogue of self-targeting nano-medical inventions to accelerate clinical trials
Samar A. Alsudir
DOI: 10.1039/D1BM00235J
Strong circularly polarized luminescence of an octahedral chromium(iii) complex
Carolin Dee, Francesco Zinna, Winald R. Kitzmann, Gennaro Pescitelli, Katja Heinze, Lorenzo Di Bari, Michael Seitz
DOI: 10.1039/C9CC06909G

![25553-77-9 - 2-[2-(哌嗪-1-基)-乙基]-1,3-二氧杂烷 25553-77-9 - 2-[2-(哌嗪-1-基)-乙基]-1,3-二氧杂烷](/structs/255/25553-77-9-5274.webp)