Dipotassium disulfate(CAS号:7790-62-7)
焦硫酸钾
基本信息
CAS号
7790-62-7
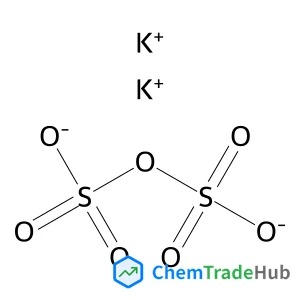
分子式
K2O7S2
分子量
254.32 g/mol
Quick Actions
基本物理性质
熔点
325 ºC
密度
2.28 g/mL at 25 °C(lit.)
溶解度
H2O: 0.1 M at 20 °C, clear, colorless
安全信息
查看安全信息危险说明
H314
危险类别
8
稳定性
Stable. Incompatible with strong acids, strong bases.
敏感性
Hygroscopic
一,将粉状硫酸钾中加入硫酸,在一定的温度下反应完毕后,冷却至50-60℃结块后,敲碎密封保存即可。 二,灼烧硫酸氢钾即可生成焦硫酸钾。在容积为500mL的瓷皿中放入500g硫酸氢钾,送入马弗炉中加热至250℃,经过30min后,将温度升高到320~340℃。硫酸氢钾开始分解起泡,并有水蒸气冒出。当停止冒泡,并有白色的SO3气体产生时,则表明KHSO4的缩聚过程结束。将所得的熔融体转移到瓷研钵中,冷却至50~60℃,趁热将其打成碎块,并迅速移入磨口瓶中用石蜡密封。 三,将硫酸氢钾加热至250℃,保持30min,升温至320~340℃,进行分解。当停止冒气泡并出现三氧化硫蒸气时,再继续加热5~10min,停止灼烧,冷却至50~60℃,敲碎熔体即得焦硫酸钾,密封保存。
同义词与参考文献
英文
- Potassium pyrosulfate
- Disulfuricacid,dipotassiumsalt
- POTASSIUM HYDROGEN SULFATE
- POTASSIUM PYROSULPHATE
- DIPOTASSIUM DISULFATE
- DI-POTASSIUM DISULPHATE
- POTASSIUM DISULFATE R. G.
- dipotassium,sulfonato sulfate
- “Anhydrous” potassiuM acid sulfate
- PotassiuM anhydrosulfate
- Potassium disulfate
- POTASSIUMPYROSULFATE
- AVJ6ZST7L6
- Disulfuric acid dipotassium salt
- dipotassium sulfonato sulfate
- Potassium pyrosulfate, 98%, for analysis
- Dipotassium disulphate
- Dipotassium pyrosulfate
- Disulfuric acid, dipotassium salt
- Potassium sulfate (K2S2O7)
- Kaliumpyrosulfat
- Potassium disulfate (K2S2O7)
- Pyrosulfuric acid, dipotassium salt
- potassium disulphate
- Disulfuric
中文
- 焦硫酸钾
- 硫酸氢钾, ACS
- 无水酸性硫酸钾
- 无水重硫酸钾
- 焦硫酸钾, 分析纯
- 水重硫酸钾
- 焦碲酸钾
- 焦硫酸钾,AR
- 焦硫酸钾,CP
- 焦硫酸钾,GR
- 二硫酸钾
MDL_Number
MFCD00011385
CAS号
7790-62-7
Merck_Index
14,7664
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海泰坦科技股份有限公司 中国 - 上海泰坦科技股份有限公司 |
|||||
 中国 - 武汉欣中科化工科技有限公司 中国 - 武汉欣中科化工科技有限公司 |
|||||
 中国 - 上海腾准生物科技有限公司 中国 - 上海腾准生物科技有限公司 |
|||||
 中国 - 江苏润丰合成科技有限公司 中国 - 江苏润丰合成科技有限公司 |
|||||
 中国 - 凌源贝卞森生物科技有限公司 中国 - 凌源贝卞森生物科技有限公司 |
|||||
 中国 - 上海金锦乐化工营销处 中国 - 上海金锦乐化工营销处 |
|||||
 德国 - STRIKO Verfahrenstechnik W.Strikfeldt & Koch GmbH 德国 - STRIKO Verfahrenstechnik W.Strikfeldt & Koch GmbH |
|||||
 中国 - 温州中树机械有限公司 中国 - 温州中树机械有限公司 |
相关文献
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Effective utilisation of waste cooking oil in a single-cylinder diesel engine using alumina nanoparticles
Sumit Roy, Pranay Kumar Parsi, R. Sreeram Kotha, Sanmitra Barman, Kalluri Vinayak, Mili Mitra Roy, Rahul Banerjee
DOI: 10.1039/C9SE00393B
In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheets
Hao Huang, Jia Li, Ya Yi, Jiahong Wang, Yihong Kang, Paul K. Chu, H. C. Ong, Xue-Feng Yu
DOI: 10.1039/C8CC00029H
Life cycle assessment of plasma-assisted ethylene production from rich-in-methane gas streams
Evangelos Delikonstantis, Elorri Igos, Michael Augustinus, Enrico Benetto
DOI: 10.1039/C9SE00736A
Water-soluble pH-switchable cobalt complexes for aqueous symmetric redox flow batteries
Yuqiao Zhou
DOI: 10.1039/D0CC00383B
PEST (political, environmental, social & technical) analysis of the development of the waste-to-energy anaerobic digestion industry in China as a representative for developing countries
Habiba Khalid, Hongyan Zhang, Caiyan Liu, Wei Li, Muhammad Khubaib Abuzar, Farrukh Raza Amin, Guangqing Liu, Chang Chen
DOI: 10.1039/C9SE00692C
Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)
DOI: 10.1039/C7CC09520A