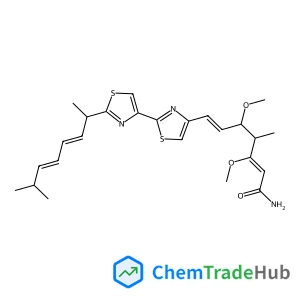
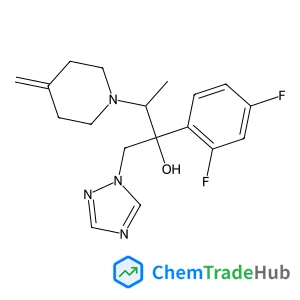
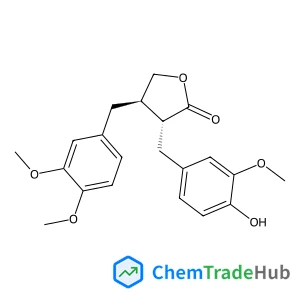
(3R,4R)-4-(3,4-Dimethoxybenzyl)-3-(4-hydroxy-3-methoxybenzyl)dihydro-2(3H)-furanone(CAS号:7770-78-7)
牛蒡子苷元
基本信息
CAS号
7770-78-7
分子式
C21H24O6
分子量
372.42 g/mol
Quick Actions
基本物理性质
物理状态
Powder
熔点
98.0 to 102.0 deg-C
沸点
567°C at 760 mmHg
密度
1.2270
闪点
198.8°C
溶解度
DMSO: 34 mg/mL with heating and sonicating, soluble
折射率
1.576
安全信息
查看安全信息危险说明
H302-H315-H319-H335
储存条件
2-8℃
同义词与参考文献
英文
- arctigenin
- 2(3H)-FURANONE,4-[(3,4-DIMETHOXYPHENYL)METHYL]DIHYDRO-3-[(4-HYDROXY-3-METHOXYPHENYL)METHYL]-,(3R,4R)
- (-)-ARCTIGENIN
- ARCTIGENIN(P)
- (-)-antirhine
- (−)-Arctigenin
- (-)-Arctigenin, Arctium lappa
- (-)-arctigenine
- (2S)-2-[(2S,12bS)-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-2-yl]but-3-en-1-ol
- (3R,4R)-4-(3,4-dimethoxybenzyl)-3-(4-hydroxy-3-methoxybenzyl)dihydrofuran-2(3H)-one
- 2-(1,2,3,4,6,7,12,12b-octahydro-indolo[2,3-a]quinolizin-2-yl)-but-3-en-1-ol
- (3R,4R)-4-[(3,4-Dimethoxyphenyl)methyl]dihydro-3-[(4-hydroxy-3-methoxyphenyl)methyl]-2(3H)-furanone
- 2(3H)-Furanone
- U76MR9VS6M
- NQWVSMVXKMHKTF-JKSUJKDBSA-N
- C21H24O6
- 2(3H)-Furanone, 4-((3,4-dimethoxyphenyl)methyl)dihydro-3-((4-hydroxy-3-methoxyphenyl)methyl)-, (3R-trans)-
- 2(3H)-Furanone, 4-((3,4-dimethoxyphenyl)methyl)dihydro-3-((4-hydroxy-3-methoxyphenyl)methyl)-, (3R,4R)-
- (3R,4R)-4-[(3,4-dimethoxyphenyl)methyl]-3-[(4-hydroxy-3-methoxyphenyl)methyl]oxolan-2-one
- (3R,4R
- (-)-Arctigenin
- Arctigenin
中文
- 牛蒡子苷元
- 牛蒡苷元
- 牛蒡甙元
- 牛蒡苷元(标准品)
- (-)-牛蒡子苷元
- 牛蒡甙元(P)
- 牛蒡子苷
- 牛蒡子苷元 植物提取物,标准品,对照品
- 牛蒡子苷元对照品
- 牛蒡苷元 标准品
- (3R,4R)-4-[(3,4-二甲氧基苯基)甲基]二氢-3-[(4-羟基-3-甲氧基苯基)甲基]-2(3H)-呋喃酮
MDL_Number
MFCD00870597
CAS号
7770-78-7
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海博飞美科化学科技有限公司 中国 - 上海博飞美科化学科技有限公司 |
|||||
 中国 - 陕西萃程生物医药科技有限公司 中国 - 陕西萃程生物医药科技有限公司 |
|||||
 中国 - 西安汇林生物科技有限公司 中国 - 西安汇林生物科技有限公司 |
|||||
 中国 - 湖北众善奉行医药科技有限公司 中国 - 湖北众善奉行医药科技有限公司 |
|||||
 中国 - 北京天誉创新科技有限公司 中国 - 北京天誉创新科技有限公司 |
|||||
 中国 - 浙江省长兴创新超细粉有限公司 中国 - 浙江省长兴创新超细粉有限公司 |
|||||
 中国 - 衡水泽浩橡胶化工有限公司 中国 - 衡水泽浩橡胶化工有限公司 |
|||||
 法国 - Verrerie Soufflée Mécanique (VSM) S.A. 法国 - Verrerie Soufflée Mécanique (VSM) S.A. |
相关文献
Developing a novel high performance NaNbO3-based lead-free dielectric capacitor for energy storage applications
DOI: 10.1039/C9SE00836E
A hollow neuronal carbon skeleton with ultrahigh pyridinic N content as a self-supporting potassium-ion battery anode
Yongwen Sun, Ya Zhang, Zheng Xing, Denghu Wei, Quanchao Zhuang
DOI: 10.1039/C9SE00889F
Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalks
Xiaobo Zhu, Shang-wen Tang, Wenyue Zhao, Xianliang Li, Zhengyi Lv, Li Yu
DOI: 10.1039/C9SE00906J
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
Engineering of electrodeposited binder-free organic-nickel hydroxide based nanohybrids for energy storage and electrocatalytic alkaline water splitting
Rohit G. Jadhav, Devraj Singh, Shaikh M. Mobin, Apurba K. Das
DOI: 10.1039/C9SE00483A
Efficient one-pot synthesis of alkyl levulinate from xylose with an integrated dehydration/transfer-hydrogenation/alcoholysis process
Mengmeng Wang, Xueying Gao, Liang He, Junhua Zhang
DOI: 10.1039/C9SE00982E
The limits to biocatalysis: pushing the envelope
Roger A. Sheldon, Dean Brady
DOI: 10.1039/C8CC02463D
Building microsphere–nanosheet structures in N-doped carbon to improve its performance in the oxygen reduction reaction and vanadium redox flow batteries
Baobing Huang, Yuchuan Liu, Miao Xia, Jiugen Qiu, Zailai Xie
DOI: 10.1039/C9SE00851A
Metal–organic frameworks: preparation and applications in highly efficient heterogeneous photocatalysis
Van-Huy Nguyen, Shi-Rong Zhou, Shu-Yu Hsu, Jia-Xuan Tan
DOI: 10.1039/C9SE00972H