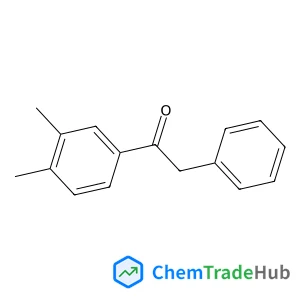
1-(3,4-Dimethylphenyl)-2-phenylethan-1-one(CAS号:65614-75-7)
基本信息
CAS号
65614-75-7
分子式
C16H16O
分子量
224.30 g/mol
Quick Actions
基本物理性质
安全信息
查看安全信息同义词与参考文献
英文
- BENZYL 3,4-DIMETHYLPHENYL KETONE
- 1-(3,4-dimethylphenyl)-2-phenylethanone
- EN300-101848
- 65614-75-7
- SCHEMBL7040136
- 1-(3,4-dimethylphenyl)-2-phenylethan-1-one
- G49659
- Z385427996
- DTXSID30374403
- CS-0229996
- AKOS002663282
- CCG-313111
MDL_Number
MFCD00156722
CAS号
65614-75-7
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 美国 - 布鲁克海文仪器公司 美国 - 布鲁克海文仪器公司 |
|||||
 中国 - 深圳市诚峰智造有限公司 中国 - 深圳市诚峰智造有限公司 |
|||||
 中国 - 戴科化学贸易化学(上海)有限公司 中国 - 戴科化学贸易化学(上海)有限公司 |
|||||
 德国 - PMR TechUG(轴承式机械设备) 德国 - PMR TechUG(轴承式机械设备) |
|||||
 中国 - 上海芯上工业科技有限公司 中国 - 上海芯上工业科技有限公司 |
|||||
 德国 - 美迪科尔特 德国 - 美迪科尔特 |
|||||
 中国 - 沈阳西凯自动化设备有限公司 中国 - 沈阳西凯自动化设备有限公司 |
相关文献
Metal–organic frameworks: preparation and applications in highly efficient heterogeneous photocatalysis
Van-Huy Nguyen, Shi-Rong Zhou, Shu-Yu Hsu, Jia-Xuan Tan
DOI: 10.1039/C9SE00972H
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cells
R. Vani, S. Ramaprabhu, Prathap Haridoss
DOI: 10.1039/C9SE01031A
Nickel-containing N-doped carbon as effective electrocatalysts for the reduction of CO2 to CO in a continuous-flow electrolyzer
Bert De Mot, Daniel Choukroun, Chen Li, Annick Hubin, Sara Bals, Jonas Hereijgers
DOI: 10.1039/C9SE00814D
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosity
An-Hua Liu, Jie-Jie Li, Bai-Hao Ren, Xin-Ru Sha, He Jiang, Xiao-Bing Lu
DOI: 10.1039/C9SE00756C
Building microsphere–nanosheet structures in N-doped carbon to improve its performance in the oxygen reduction reaction and vanadium redox flow batteries
Baobing Huang, Yuchuan Liu, Miao Xia, Jiugen Qiu, Zailai Xie
DOI: 10.1039/C9SE00851A
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
Boronic acid liposomes for cellular delivery and content release driven by carbohydrate binding‡
Xiaoyu Zhang, Daiane S. Alves, Jinchao Lou, Shelby D. Hill, Francisco N. Barrera, Michael D. Best
DOI: 10.1039/C8CC00820E
Engineering of electrodeposited binder-free organic-nickel hydroxide based nanohybrids for energy storage and electrocatalytic alkaline water splitting
Rohit G. Jadhav, Devraj Singh, Shaikh M. Mobin, Apurba K. Das
DOI: 10.1039/C9SE00483A