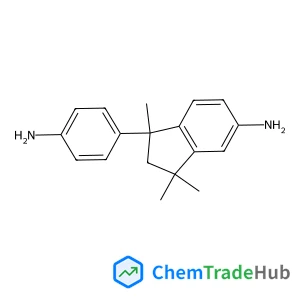
1-(4-Aminophenyl)-1,3,3-trimethyl-5-indanamine(CAS号:54628-89-6)
5(6)-氨基-1-(4-氨基苯基)-1,3,3-三甲基茚满
基本信息
CAS号
54628-89-6
分子式
C18H22N2
分子量
266.39 g/mol
Quick Actions
基本物理性质
沸点
432.3°C at 760 mmHg
密度
1.091±0.06 g/cm3 (20 ºC 760 Torr),
闪点
257.9°C
折射率
1.615
安全信息
查看安全信息同义词与参考文献
英文
- 1-(4-Aminophenyl)-2,3-dihydro-1,3,3-trimethyl-1H-inden-5-amine
- 1H-Inden-5-amine,1-(4-aminophenyl)-2,3-dihydro-1,3,3-trimethyl-
- MFCD18971260
- 1-(4-aminophenyl)-1,3,3-trimethyl-2H-inden-5-amine
- FT-0774684
- SCHEMBL137367
- 1-(4-Aminophenyl)-1,3,3-trimethyl-5-indanamine
- BCP29464
- NS00056278
- 1H-Inden-5-amine, 1-(4-aminophenyl)-2,3-dihydro-1,3,3-trimethyl-
- EINECS 259-261-6
- 1-(4-Aminophenyl)-1,3,3-trimethyl-2,3-dihydro-1H-inden-5-amine
- 54628-89-6
- DTXSID30969898
- PIDA
- 1-(4-aminophenyl)-1,3,3-trimethyl-2,3-dihydro-1H-inden-5-amine
中文
- 5(6)-氨基-1-(4-氨基苯基)-1,3,3-三甲基茚满
- 1-(4-氨基苯基)-2,3-二氢-1,3,3-三甲基 -1H-茚-5-胺
- 5(6)-1-(4-氨基苯基)-1,3,3-三甲基茚满
- 1-(4-氨基苯基)-2,3-二氢-1,3,3-三甲基-1H-茚-5-胺
MDL_Number
MFCD18971260
CAS号
54628-89-6
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 湖北清北云研医药科技有限责任公司 中国 - 湖北清北云研医药科技有限责任公司 |
|||||
 中国 - 湖北拓邦化工有限公司 中国 - 湖北拓邦化工有限公司 |
|||||
 中国 - 南通众合化工新材料有限公司 中国 - 南通众合化工新材料有限公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 陕西信瑞生物科技有限公司 中国 - 陕西信瑞生物科技有限公司 |
|||||
 中国 - 南京易普易达科技发展有限公司 中国 - 南京易普易达科技发展有限公司 |
|||||
 德国 - Elektrowärme Gabbey 德国 - Elektrowärme Gabbey |
|||||
 中国 - 上海卡恩医药科技有限公司 中国 - 上海卡恩医药科技有限公司 |
相关文献
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Kan Zhang, Ming-Ming Yang, Shan Xu, Hua-Ming Sun, Jin-Lei Zhang, Zi-Wei Gao, Wei-Qiang Zhang
DOI: 10.1039/C8CC00324F
From zinco(ii) arsaketenes to silylene-stabilised zinco arsinidene complexes
Ernesto Ballestero-Martínez, Terrance J. Hadlington, Tibor Szilvási, Shenglai Yao, Matthias Driess
DOI: 10.1039/C8CC01928B
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A
Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloaddition
Renzeng Chen, Danbo Wang, Wenbo Hao, Feng Shao, Yingjie Zhao
DOI: 10.1039/D1CC02179F
Chemoproteomics-based target profiling of sinomenine reveals multiple protein regulators of inflammation
Lianguo Chen, Hong-jian Wang, Teng-fei Ji, Chong-Jing Zhang
DOI: 10.1039/D1CC01522B
In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheets
Hao Huang, Jia Li, Ya Yi, Jiahong Wang, Yihong Kang, Paul K. Chu, H. C. Ong, Xue-Feng Yu
DOI: 10.1039/C8CC00029H
Metal–organic frameworks: preparation and applications in highly efficient heterogeneous photocatalysis
Van-Huy Nguyen, Shi-Rong Zhou, Shu-Yu Hsu, Jia-Xuan Tan
DOI: 10.1039/C9SE00972H
Biomaterials Science Emerging Investigators 2021
Maria E. Southall
DOI: 10.1039/D1BM90053F
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structure
Li-Ping Cheng, Zhi Wang, Qiao-Yu Wu, Hai-Feng Su, Tao Peng, Geng-Geng Luo, Yan-An Li, Di Sun, Lan-Sun Zheng
DOI: 10.1039/C8CC00014J