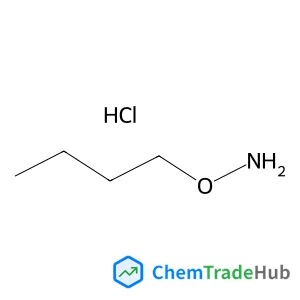
O-butylhydroxylamine hydrochloride(CAS号:4490-82-8)
i>-丁基羟胺盐酸盐
基本信息
CAS号
4490-82-8
分子式
C4H12ClNO
分子量
125.60 g/mol
Quick Actions
基本物理性质
熔点
157.0 to 161.0 deg-C
安全信息
查看安全信息危险说明
H315-H319
同义词与参考文献
英文
- O-Butylhydroxylamine hydrochloride
- <i>O<
- Aminooxy-n-butane hydrochloride
- butoxamine hydrochloride
- butoxyamine hydrochloride
- Hydroxylamine,O-butyl-,hydrochloride
- n-butyloxyamine hydrochloride
- O-Butyl-hydroxylamin,Hydrochlorid
- O-butyl-hydroxylamine,hydrochloride
- O-n-butyl-hydroxylamine hydrochloride
- n-Butoxyamine hydrochloride
- O-BUTYLHYDROXYLAMINE HCL
- Hydroxylamine, O-butyl-, hydrochloride
- butoxylamine hydrochloride
- O-ButylhydroxylamineHydrochloride
- HUYRNQWVAPCTQZ-UHFFFAOYSA-N
- TRA0052979
- SY019069
- AB0055209
- AX8232347
- V6009
- B5686
- EN300-211436
- O-butylhydroxylamine pound nothydrochloride
- O-Butyl-Hydroxylamine
- SCHEMBL242691
- CS-0106180
- MFCD00487631
- n-butoxyamine hydrochloride
- O-butylhydroxylamine;hydrochloride
- AS-17987
- O-butylhydroxylamine hydrochloride
- AKOS022184155
- DTXSID90484274
- 4490-82-8
- DA-18009
中文
- O-丁基羟胺盐酸盐
- O-丁氧胺盐酸盐
- i>-丁基羟胺盐酸盐
MDL_Number
MFCD00487631
CAS号
4490-82-8
Customs_Code
2922199090
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海捷世凯生物科技有限公司 中国 - 上海捷世凯生物科技有限公司 |
|||||
 中国 - 上海腾准生物科技有限公司 中国 - 上海腾准生物科技有限公司 |
|||||
 中国 - 湖北汉威化工有限公司 中国 - 湖北汉威化工有限公司 |
|||||
 中国 - 金锦乐(湖南)化学有限公司 中国 - 金锦乐(湖南)化学有限公司 |
|||||
 中国 - 上海阿拉丁生化科技股份有限公司 中国 - 上海阿拉丁生化科技股份有限公司 |
|||||
 中国 - 江苏金沃新材料有限公司 中国 - 江苏金沃新材料有限公司 |
|||||
 中国 - 武汉八颗星生物科技有限公司 中国 - 武汉八颗星生物科技有限公司 |
|||||
 德国 - Verfaðstechnik Schweitzer GmbH 德国 - Verfaðstechnik Schweitzer GmbH |
期刊推荐

Corrosion Science

Advances in Colloid and Interface Science

Ferroelectrics

Accounts of Chemical Research

Australian Journal of Chemistry

Chemistry of Heterocyclic Compounds

Cement and Concrete Research

Bulletin of the Chemical Society of Japan

Anti-Corrosion Methods and Materials

Chemical & Pharmaceutical Bulletin
相关文献
Three-terminal III–V/Si tandem solar cells enabled by a transparent conductive adhesive
Manuel Schnabel, Michael Rienäcker, Emily L. Warren, Paul F. Ndione, Bill Nemeth, Talysa R. Klein, Maikel F. A. M. van Hest, John F. Geisz, Robby Peibst, Paul Stradins, Adele C. Tamboli
DOI: 10.1039/C9SE00893D
Engineering of electrodeposited binder-free organic-nickel hydroxide based nanohybrids for energy storage and electrocatalytic alkaline water splitting
Rohit G. Jadhav, Devraj Singh, Shaikh M. Mobin, Apurba K. Das
DOI: 10.1039/C9SE00483A
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F
Electrocatalytic cleavage of lignin model dimers using ruthenium supported on activated carbon cloth
Mahlet Garedew, Daniel Young-Farhat, Souful Bhatia, Pengchao Hao, James E. Jackson
DOI: 10.1039/C9SE00912D
An improved fluorescent protein-based expression reporter system that utilizes bioluminescence resonance energy transfer and peptide-assisted complementation
Akira Takai, Keiko Yoshizawa
DOI: 10.1039/C9CC08664A
Carbon-based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels
Mufeedah Muringa Kandy
DOI: 10.1039/C9SE00827F
Synthesis of aviation fuel from bio-derived isophorone
Courtney Ford Ryan, Cameron M. Moore, Juan H. Leal, Troy A. Semelsberger, Jenny K. Banh, Junqing Zhu, Charles S. McEnally, Lisa D. Pfefferle, Andrew D. Sutton
DOI: 10.1039/C9SE01014A
Recent developments in carbon nitride based films for photoelectrochemical water splitting
Rui-Qin Zhang
DOI: 10.1039/C9SE00785G
Efficient one-pot synthesis of alkyl levulinate from xylose with an integrated dehydration/transfer-hydrogenation/alcoholysis process
Mengmeng Wang, Xueying Gao, Liang He, Junhua Zhang
DOI: 10.1039/C9SE00982E
Performance of electrode-supported silica membrane separators in lithium-ion batteries
Kishen Rafiz, Y. Jin, Y. S. Lin
DOI: 10.1039/C9SE00826H