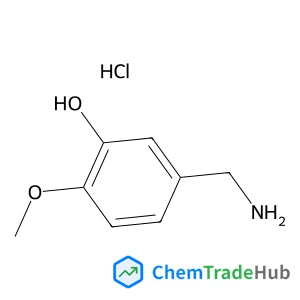
5-(Aminomethyl)-2-methoxyphenol hydrochloride (1:1)(CAS号:42365-68-4)
3-羟基-4-甲氧基苄胺盐酸盐
基本信息
CAS号
42365-68-4
分子式
C8H12ClNO2
分子量
189.64 g/mol
Quick Actions
基本物理性质
熔点
190-195 °C (lit.)
沸点
295.3°C at 760 mmHg
密度
1.161
闪点
132.4°C
折射率
1.57
安全信息
查看安全信息危险说明
H317-H319
同义词与参考文献
英文
- AKOS016003229
- AS-76450
- E77198
- 42365-68-4
- 4-methoxy-3-hydroxybenzylamine hydrochloride
- IEVBKUSXLSVMOB-UHFFFAOYSA-N
- Z415728036
- SY109944
- 3-Hydroxy-4-methoxybenzylamine hydrochloride, 97%
- 5-(aminomethyl)-2-methoxyphenol;hydrochloride
- isovanillylamine hydrochloride
- DTXSID30584469
- CS-0150581
- 3-hydroxy-4-methoxy-benzylamine hydrogen chloride
- 3-Hydroxy-4-methoxybenzylamine hydrochloride
- 3-HYDROXY-4-METHOXYBENZYLAMINEHYDROCHLORIDE
- 3-Hydroxy-4-methoxybenzylamine HCl
- 5-(Aminomethyl)-2-methoxyphenol--hydrogen chloride (1/1)
- 5-(aminomethyl)-2-methoxyphenol hydrochloride
- SCHEMBL1143719
- MFCD05664377
- 5-(aminomethyl)-2-methoxyphenolhydrochloride
- 5-aminomethyl-2-methoxy-phenol hydrochloride
- EN300-40869
- FT-0695225
- 5-(Aminomethyl)-2-methoxyphenol hydrochloride
- 3-Hydroxy-4-Methoxybenzylamine Hcl
- Phenol,5-(aminomethyl)-2-methoxy-, hydrochloride (1:1)
- 5-Aminomethyl-2-methoxy-phenol,Hydrochlorid
中文
- 3-羟基-4-甲氧基苄胺盐酸盐
MDL_Number
MFCD05664377
CAS号
42365-68-4
Customs_Code
2922509090
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海颖心实验室设备有限公司 中国 - 上海颖心实验室设备有限公司 |
|||||
 中国 - 上海捷世凯生物科技有限公司 中国 - 上海捷世凯生物科技有限公司 |
|||||
 中国 - 上海腾准生物科技有限公司 中国 - 上海腾准生物科技有限公司 |
|||||
 中国 - 湖北拓邦化工有限公司 中国 - 湖北拓邦化工有限公司 |
|||||
 中国 - 上海阿拉丁生化科技股份有限公司 中国 - 上海阿拉丁生化科技股份有限公司 |
|||||
 中国 - 上海源叶生物科技有限公司 中国 - 上海源叶生物科技有限公司 |
|||||
 德国 - 帝斯曼电脑股份公司 德国 - 帝斯曼电脑股份公司 |
|||||
 中国 - 台州市椒江天一化工厂 中国 - 台州市椒江天一化工厂 |
相关文献
Recent developments in carbon nitride based films for photoelectrochemical water splitting
Rui-Qin Zhang
DOI: 10.1039/C9SE00785G
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Permselective ion electrosorption of subnanometer pores at high molar strength enables capacitive deionization of saline water
Luca Cervini
DOI: 10.1039/C9SE00996E
Solventless thermal crosslinked polymer protective layer for high stable lithium metal batteries
Hyunjin Kim, Jeeyoung Yoo
DOI: 10.1039/C9SE01046G
Stabilizing synthetic DNA for long-term data storage with earth alkaline salts
A. Xavier Kohll, Philipp L. Antkowiak, Weida D. Chen, Bichlien H. Nguyen, Wendelin J. Stark, Luis Ceze, Karin Strauss, Robert N. Grass
DOI: 10.1039/D0CC00222D
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
Performance of electrode-supported silica membrane separators in lithium-ion batteries
Kishen Rafiz, Y. Jin, Y. S. Lin
DOI: 10.1039/C9SE00826H


![224-53-3 - 二苯并[C,H]吖啶 224-53-3 - 二苯并[C,H]吖啶](/structs/224/224-53-3-97c9.webp)