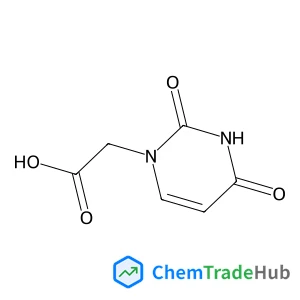
(2,4-Dioxo-3,4-dihydro-1(2H)-pyrimidinyl)acetic acid(CAS号:4113-97-7)
2-(2,4-二氧代嘧啶-1-基)乙酸
基本信息
CAS号
4113-97-7
分子式
C6H6N2O4
分子量
170.12 g/mol
Quick Actions
基本物理性质
沸点
°Cat760mmHg
密度
1.518
闪点
°C
折射率
1.557
安全信息
查看安全信息同义词与参考文献
英文
- SCHEMBL2899640
- SY151956
- SB57276
- NSC81466
- uracil-1-acetic acid
- 4113-97-7
- HMS1692B15
- 1(2h)-pyrimidineacetic acid, 3,4-dihydro-2,4-dioxo-
- (2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)acetic acid
- Dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetic acid
- 2-(2,4-dioxopyrimidin-1-yl)aceticacid
- 2-(2,4-dioxopyrimidin-1-yl)acetic acid
- CCG-40463
- CS-0149767
- FT-0769263
- (2,4-Dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-acetic acid, AldrichCPR
- (2,4-Dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-ac etic acid
- 2-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)aceticacid
- 1-(Carboxymethyl)uracil
- BS-13579
- (4-Hydroxy-2-oxopyrimidin-1(2H)-yl)acetic acid
- DTXSID10961451
- Z104502076
- AKOS000301102
- BB 0237711
- MFCD00458007
- F2158-1780
- (2,4-dioxo-3,4-dihydro-2 h-pyrimidin-1-yl)-acetic acid
- 2-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetic acid
- AC8863
- 2-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)acetic acid
- NSC-81466
- EN300-13287
- ZFNQFXDDQAEAFI-UHFFFAOYSA-N
- (2,4-dioxo-3,4-dihydro-1(2H)-pyrimidinyl)acetic acid
- (2,4-Dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-acetic acid
- (2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetic acid
- 2-(2,4-Dioxo-3,4-dihydropyrimidin-1(2H)-yl)acetic acid
- 1(2H)-Pyrimidineaceticacid, 3,4-dihydro-2,4-dioxo-
- (2,4-dioxo-3,4-dihydro-1(2H)-pyrimidinyl)acetic acid(SALTDATA: FREE)
- 1-(carboxymethyl)uracil
- 1-carboxymethyl uracil
- 2-(2,4-dioxo-1,2,3,4-tetrahydro-1-pyrimidinyl)acetic acid
- N-1-carboxymethyluracil
- Nsc81466
- Uracil-1-acetic acid
- uracil-1-ylacetic acid
- uracilacetic acid
中文
- (2,4-二氧代-3,4-二氢-2H-嘧啶-1-基)-乙酸
- 2-(2,4-二氧代嘧啶-1-基)乙酸
MDL_Number
MFCD00458007
CAS号
4113-97-7
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海颖心实验室设备有限公司 中国 - 上海颖心实验室设备有限公司 |
|||||
 中国 - 上海捷世凯生物科技有限公司 中国 - 上海捷世凯生物科技有限公司 |
|||||
 中国 - 上海腾准生物科技有限公司 中国 - 上海腾准生物科技有限公司 |
|||||
 中国 - 乐研试剂 中国 - 乐研试剂 |
|||||
 中国 - 上海绩祥生物科技有限公司 中国 - 上海绩祥生物科技有限公司 |
|||||
 中国 - 湖北实兴化工有限公司 中国 - 湖北实兴化工有限公司 |
|||||
 中国 - 潍坊昌盛硝盐有限公司 中国 - 潍坊昌盛硝盐有限公司 |
|||||
 中国 - 武汉博欧特生物科技有限公司 中国 - 武汉博欧特生物科技有限公司 |
相关文献
High-performance tungsten carbide electrocatalysts for the hydrogen evolution reaction
Jing Li, Bao Wang, Wei Liu
DOI: 10.1039/C9SE00853E
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H
Synthesis of aviation fuel from bio-derived isophorone
Courtney Ford Ryan, Cameron M. Moore, Juan H. Leal, Troy A. Semelsberger, Jenny K. Banh, Junqing Zhu, Charles S. McEnally, Lisa D. Pfefferle, Andrew D. Sutton
DOI: 10.1039/C9SE01014A
Boronic acid liposomes for cellular delivery and content release driven by carbohydrate binding‡
Xiaoyu Zhang, Daiane S. Alves, Jinchao Lou, Shelby D. Hill, Francisco N. Barrera, Michael D. Best
DOI: 10.1039/C8CC00820E
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J
Redox responsive Pluronic micelle mediated delivery of functional siRNA: a modular nano-assembly for targeted delivery
Sandeep Kadekar, Ganesh N. Nawale, Vadim Le Joncour, Pirjo Laakkonen, Jöns Hilborn, Oommen P. Varghese, Oommen P. Oommen
DOI: 10.1039/D1BM00428J
Ultra-thin NiFeSe nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting
Yu-Yang Sun, Mei-Yan Jiang, Guang-Ya Hou, Yi-Ping Tang, Min Liu
DOI: 10.1039/C9SE00905A

![28443-57-4 - 4-[4-[(5S)-5-(氨基甲基)-2-氧代-3-恶唑烷基]苯基]-3-吗啉酮 28443-57-4 - 4-[4-[(5S)-5-(氨基甲基)-2-氧代-3-恶唑烷基]苯基]-3-吗啉酮](/structs/284/28443-57-4-e4c7.webp)