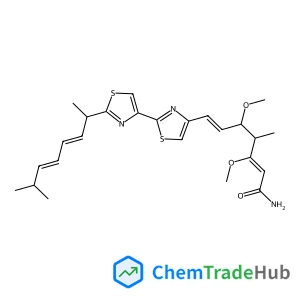
Amphotericin B Methyl Ester(CAS号:36148-89-7)
两性霉素B甲酯
基本信息

CAS号
36148-89-7
分子式
C48H75NO17
分子量
938.12 g/mol
Quick Actions
基本物理性质
沸点
1102.6°Cat760mmHg
闪点
620.6°C
折射率
1.602
安全信息
查看安全信息同义词与参考文献
英文
- UNII-074Z98YIW3
- 074Z98YIW3
- SCHEMBL20988147
- Methylamphotericin B
- UAZIZEMIKKIBCA-TYVGYKFWSA-N
- Amphotericin B Methyl Ester 90per cent
- CHEBI:277842
- Q27225718
- 36148-89-7
- CHEMBL321176
- methyl (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylate
- Amphotericin B Methyl Ester 90%
- methyl (1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(3-amino-3,6-dideoxy-beta-D-mannopyranosyl)oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylate
- CS-0111429
- HY-135327
- DTXSID701009331
- AMPHOTERICIN B METHYL ESTER
- Amphotericin B Me Ester
- Amphotericin B, methylester
- Amb
- METHYLAMPHOTERICIN B
- Amphotericin B methyl ester
- methyl(1R,3S,5R,6R,9R,11R,15S,16R,17R,18S,19E,21E,23E,25E,27E,29E,31E,33R,35S,36R,37S)-33-(((2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-1,3,5,6,9,11,17,37-octahydroxy-
中文
- 甲基(1R,3S,5S,8S,9S,11R,15S,16S,17R,18S,25Z,33R,35S,36R,37S)-33-[(3-氨基-3,6-二脱氧-beta-D-甘露糖基)氧基]-1,3,5,8,9,11,17,37-八羟基-15,16,18-三甲基-13-氧代-14,39-二氧杂双环[33.3.1]N酮a三十碳-19,21,23,
- 两性霉素B-13C6甲酯
- 两性霉素B甲酯
MDL_Number
MFCD30719782
CAS号
36148-89-7
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海陌孚医药科技有限公司 中国 - 上海陌孚医药科技有限公司 |
|||||
 中国 - 湖北省德奥化研医药科技有限责任公司 中国 - 湖北省德奥化研医药科技有限责任公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 上海绩祥生物科技有限公司 中国 - 上海绩祥生物科技有限公司 |
|||||
 中国 - 上海阿拉丁生化科技股份有限公司 中国 - 上海阿拉丁生化科技股份有限公司 |
|||||
 中国 - 上海祥韦思化学品有限公司 中国 - 上海祥韦思化学品有限公司 |
|||||
 中国 - 云南氟业 中国 - 云南氟业 |
|||||
 中国 - 青岛鑫晟泰生物科技有限公司 中国 - 青岛鑫晟泰生物科技有限公司 |
相关文献
Water-soluble pH-switchable cobalt complexes for aqueous symmetric redox flow batteries
Yuqiao Zhou
DOI: 10.1039/D0CC00383B
Vapor-fed photoelectrolysis of water at 0.3 V using gas-diffusion photoanodes of SrTiO3 layers
Hyosuke Mukohara, Hiroki Sato, Chihiro Tateishi, Hiromasa Sato
DOI: 10.1039/C9SE01068H
Novel aqueous amine looping approach for the direct capture, conversion and storage of CO2 to produce magnesium carbonate
Meishen Liu, Hassnain Asgar, Soenke Seifert, Greeshma Gadikota
DOI: 10.1039/C9SE00316A
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F
Biomaterials Science Emerging Investigators 2021
Maria E. Southall
DOI: 10.1039/D1BM90053F
Palladium-catalyzed silaborative carbocyclizations of 1,6-diynes
Qian Zhang, Qiu-Ju Liang, Jian-Lin Xu, Yun-He Xu
DOI: 10.1039/C8CC00097B
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring system
Huamin Chen, Chao Xing, Yuliang Li, Jun Wang
DOI: 10.1039/C9SE01184F
From Douglas fir to renewable H2-enriched syngas via ex situ catalytic pyrolysis over metal nanoparticles–nanocellulose derived carbon catalysts
Hanwu Lei, Chenxi Wang, Moriko Qian, Elmar Villota, Wendy Mateo
DOI: 10.1039/C9SE00860H
Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloaddition
Renzeng Chen, Danbo Wang, Wenbo Hao, Feng Shao, Yingjie Zhao
DOI: 10.1039/D1CC02179F
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A