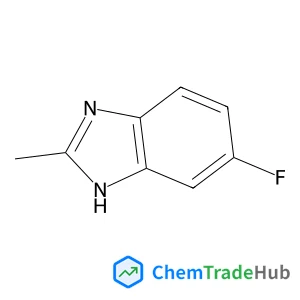
1-Phenoxy-2-propanamine(CAS号:35205-54-0)
乙胺,1-甲基-2-苯氧基-
基本信息

CAS号
35205-54-0
分子式
C9H13NO
分子量
151.21 g/mol
Quick Actions
基本物理性质
沸点
240.5°Cat760mmHg
密度
1.004
闪点
99.5°C
折射率
1.519
安全信息
查看安全信息危险类别
IRRITANT
同义词与参考文献
英文
- NSC137777
- (1-methyl-2-phenoxyethyl)amine
- LS-09259
- Ethylamine, methyl-2-phenoxy-
- SB76412
- 2-Propanamine, 1-phenoxy-
- 35205-54-0
- CS-0433311
- 4-06-00-00669 (Beilstein Handbook Reference)
- ETHYLAMINE, 1-METHYL-2-PHENOXY-
- 1-methyl-2-phenoxyethyl amine
- C 1926
- Phenoxyisopropylamine
- EN300-58378
- BB 0255191
- MFCD00008083
- 1-Methyl-2-phenoxy-ethylamine
- N12753
- .alpha.-Methyl-2-phenoxyethanamine
- NSC-137777
- 1-Phenoxy-2-propylamine
- 2-Phenoxy-1-methylethylamine
- SCHEMBL366154
- CHEMBL162135
- AB01007136-01
- 1-Phenoxypropane-2-amine
- LS-68252
- 1-Phenoxy-2-propanamine
- NSC 137777
- AKOS016042140
- 1-phenoxypropan-2-amine
- FT-0678798
- BRN 2045304
- 1-Methyl-2-phenoxyethylamine
- 2-Amino-1-phenoxypropane
- O(CC(C)N)c1ccccc1
- DTXSID301313534
- AKOS000302300
- 2-Phenoxyisopropylamine
- EINECS 252-434-7
- 1-Phenoxypropan-2-amine
- (2R)-1-phenoxypropan-2-amine
- 1-METHYL-2-PHENOXY-ETHYLAMINE
- Ethylamine, 1-methyl-2-phenoxy-
- α-Methyl-2-phenoxyethanamine
中文
- 1-(苯氧基)丙-2-胺
- 1-甲基-2-苯氧基乙胺
- 乙胺,1-甲基-2-苯氧基-
MDL_Number
MFCD00008083
CAS号
35205-54-0
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 湖北成海化工有限公司 中国 - 湖北成海化工有限公司 |
|||||
 中国 - 湖北达豪化工有限公司 中国 - 湖北达豪化工有限公司 |
|||||
 中国 - 湖北拓邦化工有限公司 中国 - 湖北拓邦化工有限公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 上海源叶生物科技有限公司 中国 - 上海源叶生物科技有限公司 |
|||||
 中国 - 石家庄天越精细化工有限公司 中国 - 石家庄天越精细化工有限公司 |
|||||
 中国 - 安徽贝意克设备技术有限公司 中国 - 安徽贝意克设备技术有限公司 |
|||||
 中国 - 深圳康宇达发光材料有限公司 中国 - 深圳康宇达发光材料有限公司 |
相关文献
Life cycle assessment of power-to-gas with biogas as the carbon source
Xiaojin Zhang, Julia Witte, Tilman Schildhauer, Christian Bauer
DOI: 10.1039/C9SE00986H
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Carbon and carbon composites obtained using deep eutectic solvents and aqueous dilutions thereof
Gaspar Carrasco-Huertas, Rafael J. Jiménez-Riobóo, María Concepción Gutiérrez, María Luisa Ferrer, Francisco del Monte
DOI: 10.1039/D0CC00681E
Novel aqueous amine looping approach for the direct capture, conversion and storage of CO2 to produce magnesium carbonate
Meishen Liu, Hassnain Asgar, Soenke Seifert, Greeshma Gadikota
DOI: 10.1039/C9SE00316A
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A
A model-based comparison of Ru and Ni catalysts for the Sabatier reaction
DOI: 10.1039/C9SE00787C
From Douglas fir to renewable H2-enriched syngas via ex situ catalytic pyrolysis over metal nanoparticles–nanocellulose derived carbon catalysts
Hanwu Lei, Chenxi Wang, Moriko Qian, Elmar Villota, Wendy Mateo
DOI: 10.1039/C9SE00860H
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structure
Li-Ping Cheng, Zhi Wang, Qiao-Yu Wu, Hai-Feng Su, Tao Peng, Geng-Geng Luo, Yan-An Li, Di Sun, Lan-Sun Zheng
DOI: 10.1039/C8CC00014J
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E

![500789-05-9 - (Betar)-2-氯-Beta-[[(1,1-二甲基乙氧基)羰基]氨基]-苯丙酸 500789-05-9 - (Betar)-2-氯-Beta-[[(1,1-二甲基乙氧基)羰基]氨基]-苯丙酸](/structs/500/500789-05-9-80b4.webp)