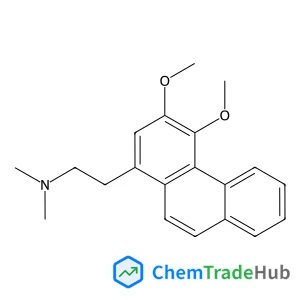
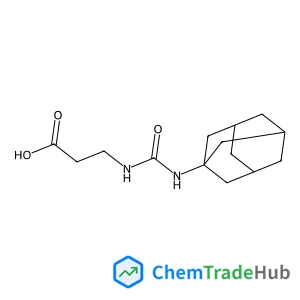
N-(Adamantan-1-ylcarbamoyl)-beta-alanine(CAS号:33205-70-8)
N-(Adamantan-1-ylcarbamoyl)-尾-alanine
基本信息
CAS号
33205-70-8
分子式
C14H22N2O3
分子量
266.34 g/mol
Quick Actions
基本物理性质
沸点
516.8°Cat760mmHg
密度
1.25
闪点
266.3°C
安全信息
查看安全信息同义词与参考文献
英文
- 3-{[(1-adamantylamino)carbonyl]amino}propanoic acid
- 33205-70-8
- 3-(1-adamantylcarbamoylamino)propanoic acid
- 3-{[(ADAMANTAN-1-YL)CARBAMOYL]AMINO}PROPANOIC ACID
- SCHEMBL7822233
- Oprea1_294866
- Oprea1_474240
- EN300-07551
- Z56757713
- 3-{[(1-ADAMANTYLAMINO)CARBONYL]AMINO}PROPANOICACID
- BRD-K21396845-001-01-9
- MFCD01791133
- HMS1608I05
- 3-([(1-ADAMANTYLAMINO)CARBONYL]AMINO)PROPANOIC ACID
- CBDivE_014941
- CS-0220780
- AKOS000200221
- N-[(1-adamantylamino)carbonyl]-beta-alanine
- 3-(3-(Adamantan-1-yl)ureido)propanoic acid
- Z380844686
- 3-{[(1-ADAMANTYLAMINO)CARBONYL]AMINO}PROPANOIC ACID
- N-[(1-adamantylamino)carbonyl]-beta-alanine(SALTDATA: FREE)
- N-[(1-adamantylamino)carbonyl]-β-alanine(SALTDATA: FREE)
中文
- 3-{[(1-金刚烷氨基)羰基]氨基}丙酸
- N-(Adamantan-1-ylcarbamoyl)-尾-alanine
MDL_Number
MFCD01791133
CAS号
33205-70-8
Customs_Code
2924299090
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 上海绩祥生物科技有限公司 中国 - 上海绩祥生物科技有限公司 |
|||||
 中国 - 上海阿拉丁生化科技股份有限公司 中国 - 上海阿拉丁生化科技股份有限公司 |
|||||
 中国 - 盐城远东化工有限公司 中国 - 盐城远东化工有限公司 |
|||||
 中国 - 上海赫诗特化工有限公司(赫特国 中国 - 上海赫诗特化工有限公司(赫特国 |
|||||
 德国 - BÜsch Technology GmbH & Co. KG 德国 - BÜsch Technology GmbH & Co. KG |
|||||
 中国 - 佛山市安你心香精香料有限公司 中国 - 佛山市安你心香精香料有限公司 |
|||||
 中国 - 武汉博欧特生物科技有限公司 中国 - 武汉博欧特生物科技有限公司 |
相关文献
Near infrared light activation of an injectable whole-cell cancer vaccine for cancer immunoprophylaxis and immunotherapy
Fei Wang, Junbin Gao, Shuanghu Wang, Jiamiao Jiang, Yicheng Ye, Juanfeng Ou, Shuwen Liu, Fei Peng, Yingfeng Tu
DOI: 10.1039/D1BM00542A
Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer's disease brain tissue
Ujjayini Ghosh, Wai-Ming Yau, Robert Tycko
DOI: 10.1039/C8CC01967C
Insights into the mechanism of photosynthetic H2 evolution catalyzed by a heptacoordinate cobalt complex
Fiorella Lucarini, Jennifer Fize, Adina Morozan, Mirco Natali, Mariachiara Pastore, Vincent Artero, Albert Ruggi
DOI: 10.1039/C9SE00434C
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
Photoactivatable fluorophores for durable labelling of individual cells
Hiroki Kashima, Mako Kamiya, Shotaro Nakano, Masayuki Miura
DOI: 10.1039/D1CC01488A
Ultra-thin NiFeSe nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting
Yu-Yang Sun, Mei-Yan Jiang, Guang-Ya Hou, Yi-Ping Tang, Min Liu
DOI: 10.1039/C9SE00905A
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A
An overview of latest advances in exploring bioactive peptide hydrogels for neural tissue engineering
Pooja Sharma, Vijay Kumar Pal, Sangita Roy
DOI: 10.1039/D0BM02049D
Performance of electrode-supported silica membrane separators in lithium-ion batteries
Kishen Rafiz, Y. Jin, Y. S. Lin
DOI: 10.1039/C9SE00826H

![224-53-3 - 二苯并[C,H]吖啶 224-53-3 - 二苯并[C,H]吖啶](/structs/224/224-53-3-97c9.webp)