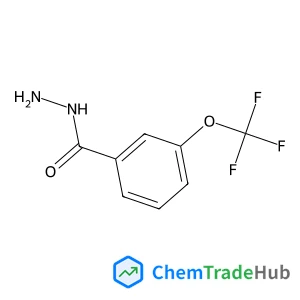
3-(Trifluoromethoxy)benzohydrazide(CAS号:321195-88-4)
3-三氟甲氧基苯甲酰肼
基本信息
CAS号
321195-88-4
分子式
C8H7F3N2O2
分子量
220.15 g/mol
Quick Actions
基本物理性质
熔点
94-96°C
安全信息
查看安全信息危险类别
IRRITANT
敏感性
Air Sensitive
同义词与参考文献
英文
- CS-0333052
- SCHEMBL679553
- 321195-88-4
- DTXSID60396618
- MFCD03425698
- 3-(Trifluoromethoxy)benzhydrazide
- FT-0676579
- A875708
- 3-(trifluoromethoxy)benzohydrazide
- SB86383
- EN300-6221684
- 3-Trifluoromethoxybenzoic acid hydrazide
- 3-trifluoromethoxy-benzoic acid hydrazide
- AKOS015853109
- 3-(Trifluoromethoxy)benzohydrazide
- 3-(TRIFLUOROMETHOXY)BENZOIC ACID HYDRAZIDE
- Benzoic acid,3-(trifluoromethoxy)-, hydrazide
中文
- 3-(三氟甲氧基)苯甲酰肼
- 3-三氟甲氧基苯甲酰肼
MDL_Number
MFCD03425698
CAS号
321195-88-4
Customs_Code
2928000090
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 上海绩祥生物科技有限公司 中国 - 上海绩祥生物科技有限公司 |
|||||
 中国 - 金锦乐(湖南)化学有限公司 中国 - 金锦乐(湖南)化学有限公司 |
|||||
 中国 - 上海阿拉丁生化科技股份有限公司 中国 - 上海阿拉丁生化科技股份有限公司 |
|||||
 中国 - 上海源叶生物科技有限公司 中国 - 上海源叶生物科技有限公司 |
|||||
 中国 - 桐乡市化工有限公司 中国 - 桐乡市化工有限公司 |
|||||
 中国 - 四川省申联生物科技有限责任公司 中国 - 四川省申联生物科技有限责任公司 |
|||||
 中国 - 山东平原四环药业有限公司 中国 - 山东平原四环药业有限公司 |
相关文献
Direct arylation polycondensation towards water/alcohol-soluble conjugated polymers as the electron transporting layers for organic solar cells
Qingwu Yin, Zhenfeng Wang, Boming Xie, Fei Huang, Yong Cao
DOI: 10.1039/D1CC01128F
Insights into the mechanism of photosynthetic H2 evolution catalyzed by a heptacoordinate cobalt complex
Fiorella Lucarini, Jennifer Fize, Adina Morozan, Mirco Natali, Mariachiara Pastore, Vincent Artero, Albert Ruggi
DOI: 10.1039/C9SE00434C
Chemoproteomics-based target profiling of sinomenine reveals multiple protein regulators of inflammation
Lianguo Chen, Hong-jian Wang, Teng-fei Ji, Chong-Jing Zhang
DOI: 10.1039/D1CC01522B
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H
Efficient one-pot synthesis of alkyl levulinate from xylose with an integrated dehydration/transfer-hydrogenation/alcoholysis process
Mengmeng Wang, Xueying Gao, Liang He, Junhua Zhang
DOI: 10.1039/C9SE00982E
Redox responsive Pluronic micelle mediated delivery of functional siRNA: a modular nano-assembly for targeted delivery
Sandeep Kadekar, Ganesh N. Nawale, Vadim Le Joncour, Pirjo Laakkonen, Jöns Hilborn, Oommen P. Varghese, Oommen P. Oommen
DOI: 10.1039/D1BM00428J
Novel aqueous amine looping approach for the direct capture, conversion and storage of CO2 to produce magnesium carbonate
Meishen Liu, Hassnain Asgar, Soenke Seifert, Greeshma Gadikota
DOI: 10.1039/C9SE00316A