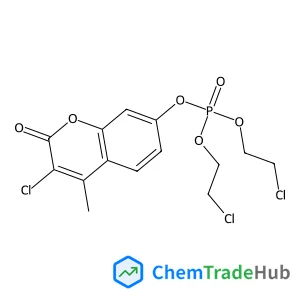
Bis(2-chloroethyl) 3-chloro-4-methyl-2-oxo-2H-chromen-7-yl phosphate(CAS号:321-55-1)
哈洛克酮标准品
基本信息
CAS号
321-55-1
分子式
C14H14Cl3O6P
分子量
415.59 g/mol
Quick Actions
基本物理性质
熔点
91°
沸点
149°C
安全信息
查看安全信息毒性
LD50 orally in rats: 900 mg/kg (Brown)
同义词与参考文献
英文
- Haloxone
- Haloxon [INN:BAN]
- NS00029219
- Tox21_111889
- CHEMBL1897362
- Haloxona [INN-Spanish]
- 3-Chloro-4-methyl-2-oxo-2H-chromen-7-yl bis(2-chloroethyl) phosphate
- HALOXON [INN]
- Tox21_111889_1
- Ethanol, 2-chloro-, hydrogen phosphate, ester with 3-chloro-7-hydroxy-4-methylcoumarin
- Ethanol, 2-chloro-, phosphate diester, ester with 3-chloro-7-hydroxy-4-methylcoumarin
- CS-3318
- Q27289815
- 3-Chloro-7-hydroxy-4-methyl-2H-1-benzopyran-2-one bis(chloroethyl)phosphate
- O,O-Di(2-chloroethyl)-O-(3-chloro-4-methylcoumarin-7-yl)phosphate
- Phosphorsaeure-O,O-di-(2-chlorethyl)-O-(3'-chlor-4'-methyl-cumarin-7-yl)ester
- phosphoric acid-bis(2-chloroethyl)(3-chloro-4-methyl-7-coumarin)
- Galloxon
- Haloxonum (INN-Latin)
- Helmirone
- NCGC00160546-01
- Di-(2-chloroethyl) 3-chloro-4-methylcoumarin-7-yl phosphate
- DB11419
- HALOXON [GREEN BOOK]
- HY-17532
- Helmiron
- Eustidil
- DTXSID5046221
- DTXCID3026221
- O,O-Bis(2-chloroethyl) O-(3-chloro-4-methyl-7-coumarinyl) phosphate
- Haloxonum [INN-Latin]
- Galoxone
- Haloxon
- Haloxonum
- SCHEMBL167194
- 3-Chloro-7-hydroxy-4-methylcoumarin bis(2-chloroethyl)phosphate
- HALOXON [MI]
- UNII-T8KXA37068
- Haloxone (INN-French)
- 2C-I(2,5-dimethoxy-4-iodophenEthylamine)
- CAS-321-55-1
- Haloxona
- SR-01000945008
- Helmirane
- 321-55-1
- Halox Wormer Drench
- Halox Bolus
- EINECS 206-289-1
- Haloxon, VETRANAL(TM), analytical standard
- SR-01000945008-1
- NCGC00160546-02
- COUMARIN, 3-CHLORO-7-HYDROXY-4-METHYL-, BIS(2-CHLOROETHYL) PHOSPHATE
- AKOS024370929
- 3-Chloro-4-methyl-umbelliferone bis(2-chloroethyl)phosphate
- BRN 1271357
- Di-(2-chloroethyl)-3-chloro-4-methyl-7-coumarinyl phosphate
- Phosphoric acid, bis(2-chloroethyl) 3-chloro-4-methyl-2-oxo-2H-1-benzopyran-7-yl ester
- Haloxona (INN-Spanish)
- 96H60
- HALOXON [MART.]
- HALOXON (MART.)
- Loxon
- bis(2-chloroethyl) (3-chloro-4-methyl-2-oxochromen-7-yl) phosphate
- AI3-50680
- T8KXA37068
- Luxon
- Haloxone [INN-French]
- Phosphoric acid,bis(2-chloroethyl) 3-chloro-4-methyl-2-oxo-2H-1-benzopyran-7-yl ester
- HALOXONA
- Ethanol, 2-chloro-, hydrogen phosphate, ester with 3-chl
中文
- 哈洛克酮
- 哈洛克酮标准品
- Haloxon
MDL_Number
MFCD00010716
CAS号
321-55-1
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海颖心实验室设备有限公司 中国 - 上海颖心实验室设备有限公司 |
|||||
 中国 - 上海泰坦科技股份有限公司 中国 - 上海泰坦科技股份有限公司 |
|||||
 中国 - 上海捷世凯生物科技有限公司 中国 - 上海捷世凯生物科技有限公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 上海源溪生物科技有限公司 中国 - 上海源溪生物科技有限公司 |
|||||
 中国 - 上海源叶生物科技有限公司 中国 - 上海源叶生物科技有限公司 |
|||||
 中国 - 常州凯康生物科技有限公司 中国 - 常州凯康生物科技有限公司 |
|||||
 德国 - 电池球 GmbH 德国 - 电池球 GmbH |
相关文献
Microscopic insights into long-range 1D ordering in a dense semi-disordered molecular overlayer
Ryan T. Hannagan, Isaac Onyango, Amanda Larson, E. Charles H. Sykes
DOI: 10.1039/D1CC01574E
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Kan Zhang, Ming-Ming Yang, Shan Xu, Hua-Ming Sun, Jin-Lei Zhang, Zi-Wei Gao, Wei-Qiang Zhang
DOI: 10.1039/C8CC00324F
Cu2ZnSnS4 nanocrystals for microwave thermal and microwave dynamic combination tumor therapy
Taya Tang, Xiaomu Xu, Zhiwen Wang, Jijing Tian, Yue Yang, Caizhang Ou, Huihui Bao, Tianlong Liu
DOI: 10.1039/C9CC07762F
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J
The limits to biocatalysis: pushing the envelope
Roger A. Sheldon, Dean Brady
DOI: 10.1039/C8CC02463D
Insights into the mechanism of photosynthetic H2 evolution catalyzed by a heptacoordinate cobalt complex
Fiorella Lucarini, Jennifer Fize, Adina Morozan, Mirco Natali, Mariachiara Pastore, Vincent Artero, Albert Ruggi
DOI: 10.1039/C9SE00434C
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H

![25553-77-9 - 2-[2-(哌嗪-1-基)-乙基]-1,3-二氧杂烷 25553-77-9 - 2-[2-(哌嗪-1-基)-乙基]-1,3-二氧杂烷](/structs/255/25553-77-9-5274.webp)
![503070-57-3 - 2-[2-(6-溴己氧基)乙氧基甲基]-1,3-二氯苯 503070-57-3 - 2-[2-(6-溴己氧基)乙氧基甲基]-1,3-二氯苯](/structs/503/503070-57-3-bc25.webp)