1,1,1,3,3,3-Hexachloro-2,2-difluoropropane(CAS号:3182-26-1)
基本信息
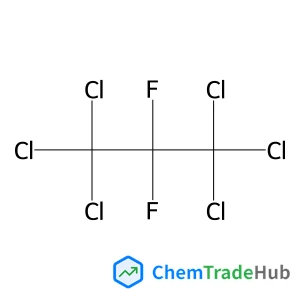
CAS号
3182-26-1
分子式
C3Cl6F2
分子量
286.75 g/mol
Quick Actions
基本物理性质
熔点
-12.7°C
沸点
196.75°C
折射率
1.4898 (estimate)
安全信息
查看安全信息同义词与参考文献
英文
- Propane, 2,2-difluoro-1,1,1,3,3,3-hexachloro-
- CCl3CF2CCl3
- NS00127082
- 4-01-00-00205 (Beilstein Handbook Reference)
- Propane, 1,1,1,3,3,3-hexachloro-2,2-difluoro-
- MFCD00018830
- 1,1,1,3,3,3-Hexachloro-2,2-difluoropropane, 97%
- 2,2-difluoro-1,1,1,3,3,3-hexachloro-propane
- BRN 1759610
- CFC-212ca
- 1,1,1,3,3,3-Hexachloro-2,2-difluoropropane
- 3182-26-1
- SCHEMBL1223422
- 2,2-Difluoro-1,1,1,3,3,3-hexachloropropane
- Hexachloro-2,2-difluoropropane
- DTXSID2074322
- Propane,1,1,1,3,3,3-hexachloro-2,2-difluoro-
- 1,1,1,3,3,3-Hexachlor-2,2-difluor-propan
- 1,1,1,3,3,3-hexachloro-2,2-difluoro-propane
- 1,1,1,3,3,3-hexachlorodifluoropropane
- 2,2-Difluoro-1,1,1,3,3,3-hexachloroprop
- AC1L2RDG
- AG-F-06185
- CTK4G7791
- difluoro-2,2-perchloropropane
- Hexachlor-2,2-difluor-propan
- hexachloro 1,1,1,3,3,3 difluoro 2,2 propane
- hexachloro-2,2-difluoro-propane
MDL_Number
MFCD00018830
CAS号
3182-26-1
推荐供应商
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 南京延乔科技有限公司 中国 - 南京延乔科技有限公司 |
|||||
 中国 - 上海开特生物科技有限公司 中国 - 上海开特生物科技有限公司 |
|||||
 德国 - Jüke Systemtechnik GmbH 德国 - Jüke Systemtechnik GmbH |
|||||
 中国 - 长沙建运有色金属有限公司 中国 - 长沙建运有色金属有限公司 |
|||||
 奥地利 - LOBA费恩化学股份公司 奥地利 - LOBA费恩化学股份公司 |
|||||
 德国 - BÜsch Technology GmbH & Co. KG 德国 - BÜsch Technology GmbH & Co. KG |
|||||
 中国 - 湖南省岳阳市云溪区道仁矶溶剂化 中国 - 湖南省岳阳市云溪区道仁矶溶剂化 |
|||||
 中国 - 深圳市中盛和实业有限公司 中国 - 深圳市中盛和实业有限公司 |
相关文献
Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cells
R. Vani, S. Ramaprabhu, Prathap Haridoss
DOI: 10.1039/C9SE01031A
Near infrared light activation of an injectable whole-cell cancer vaccine for cancer immunoprophylaxis and immunotherapy
Fei Wang, Junbin Gao, Shuanghu Wang, Jiamiao Jiang, Yicheng Ye, Juanfeng Ou, Shuwen Liu, Fei Peng, Yingfeng Tu
DOI: 10.1039/D1BM00542A
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
Building microsphere–nanosheet structures in N-doped carbon to improve its performance in the oxygen reduction reaction and vanadium redox flow batteries
Baobing Huang, Yuchuan Liu, Miao Xia, Jiugen Qiu, Zailai Xie
DOI: 10.1039/C9SE00851A
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
Increasing efficiency of perovskite solar cells using low concentrating photovoltaic systems
Hasan Baig, Hiroyuki Kanda, Abdullah M. Asiri, Mohammad Khaja Nazeeruddin, Tapas Mallick
DOI: 10.1039/C9SE00550A
Novel aqueous amine looping approach for the direct capture, conversion and storage of CO2 to produce magnesium carbonate
Meishen Liu, Hassnain Asgar, Soenke Seifert, Greeshma Gadikota
DOI: 10.1039/C9SE00316A
Water-soluble pH-switchable cobalt complexes for aqueous symmetric redox flow batteries
Yuqiao Zhou
DOI: 10.1039/D0CC00383B
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosity
An-Hua Liu, Jie-Jie Li, Bai-Hao Ren, Xin-Ru Sha, He Jiang, Xiao-Bing Lu
DOI: 10.1039/C9SE00756C
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B