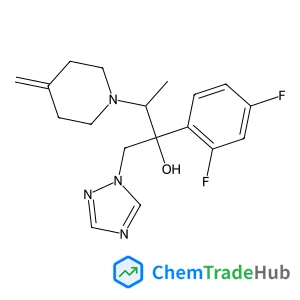
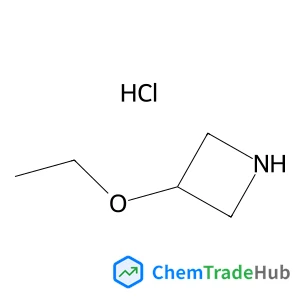
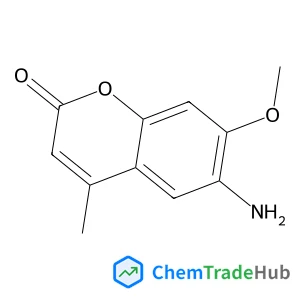
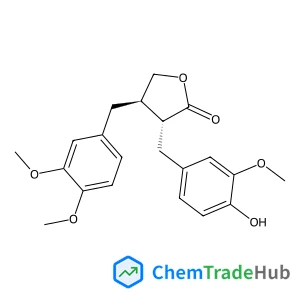
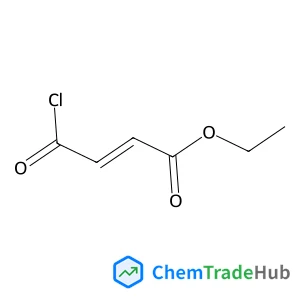
Ethyl (2E)-4-chloro-4-oxo-2-butenoate(CAS号:26367-48-6)
富马酸单乙酯酰氯
基本信息
CAS号
26367-48-6
分子式
C6H7ClO3
分子量
162.57 g/mol
Quick Actions
基本物理性质
物理状态
No data available
熔点
No data available
沸点
218 ºC
闪点
93 ºC
折射率
n20/D 1.463
安全信息
查看安全信息危险说明
H302-H314-H317-H334
敏感性
Sensitive to moisture
同义词与参考文献
英文
- EN300-1721902
- Ethyl fumaroyl chloride
- EN300-262026
- YYLWXDIGYFPUSK-ONEGZZNKSA-N
- ethyl (E)-3-chlorocarbonylacrylate
- (E)-Ethyl4-chloro-4-oxobut-2-enoate
- Ethyl fumaryl chloride
- J-016397
- 3-CHLOROCARBONYLACRYLIC ACID ETHYL ESTER
- Ethyl fumaroyl chloride, >=97.0%
- Ethyl 4-chloro-4-oxobut-2-enoate
- MFCD03002066
- 66130-92-5
- (E)-3-chlorocarbonyl acrylic acid ethyl ester
- AS-77194
- (E)-Ethyl 4-chloro-4-oxobut-2-enoate
- ethyl trans-3-(chloroformyl)acrylate
- 2-Butenoic acid, 4-chloro-4-oxo-, ethyl ester, (2E)-
- 26367-48-6
- AKOS015902225
- (E)-3-(Chloroformyl)-acrylic acid ethyl ester
- E78218
- ethyl (2E)-4-chloro-4-oxobut-2-enoate
- SCHEMBL1418555
- BBA36748
- ethyl (E)-4-chloro-4-oxobut-2-enoate
- Ethylfumaroylchloride
- 3-Chlorocarbonylacrylic acid ethyl ester
- (E)-4-Chloro-4-oxo-2-butenoic acid ethyl ester
- Ethyl fumaryl
- FUMARIC ACID MONOETHYL ESTER HYDROCHLORIDE
- (E)-3-(Chloroformyl)-acr
- (E)-3-ethoxycarbonyl-2-propenoyl chloride
- 3-(ethyloxycarbonyl)propenoyl chloride
- 3-Chlorocarbonylacrilicacid ethylester
- 3-chlorocarbonyl-acrylic acid ethyl ester
- ethyl 3-chlorocarbonylacrylate
- fumaryl chloride monoethyl ester
- trans-3-ethoxycarbonyl-2-propenoyl chloride
- (Fumaric acid 1-ethyl) chloride
- V0861
- 4-Chloro-4-oxo-2-butenoic
中文
- 富马酸单乙酯酰氯
- (E)-3-(氯甲酰)-丙烯酸乙酯
MDL_Number
MFCD03002066
CAS号
26367-48-6
Customs_Code
2918300090
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 湖北汉威化工有限公司 中国 - 湖北汉威化工有限公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 上海绩祥生物科技有限公司 中国 - 上海绩祥生物科技有限公司 |
|||||
 中国 - 江苏润丰合成科技有限公司 中国 - 江苏润丰合成科技有限公司 |
|||||
 中国 - 上海阿拉丁生化科技股份有限公司 中国 - 上海阿拉丁生化科技股份有限公司 |
|||||
 中国 - 上海优拓医药科技有限公司 中国 - 上海优拓医药科技有限公司 |
|||||
 中国 - 力码(广州)材料科技有限公司 中国 - 力码(广州)材料科技有限公司 |
|||||
 中国 - 郑州联创食化工贸有限公司 中国 - 郑州联创食化工贸有限公司 |
相关文献
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H
Catalogue of self-targeting nano-medical inventions to accelerate clinical trials
Samar A. Alsudir
DOI: 10.1039/D1BM00235J
A model-based comparison of Ru and Ni catalysts for the Sabatier reaction
DOI: 10.1039/C9SE00787C
Catalytic depolymerization of Kraft lignin to produce liquid fuels via Ni–Sn metal oxide catalysts
Baikai Zhang, Wenzhi Li, Xiaomeng Dou, Jindong Wang, Lele Jin, Ajibola T. Ogunbiyi, Xiaosen Li
DOI: 10.1039/C9SE01089K
Building microsphere–nanosheet structures in N-doped carbon to improve its performance in the oxygen reduction reaction and vanadium redox flow batteries
Baobing Huang, Yuchuan Liu, Miao Xia, Jiugen Qiu, Zailai Xie
DOI: 10.1039/C9SE00851A
In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheets
Hao Huang, Jia Li, Ya Yi, Jiahong Wang, Yihong Kang, Paul K. Chu, H. C. Ong, Xue-Feng Yu
DOI: 10.1039/C8CC00029H
An environmentally friendly natural polymer as a universal interfacial modifier for fullerene and non-fullerene polymer solar cells
Xiaojing Wang, Shuwang Yi, Zhicai He, Xinhua Ouyang, Hong-Bin Wu, Weiguo Zhu, Bin Zhang, Yong Cao
DOI: 10.1039/C9SE01079C
Permselective ion electrosorption of subnanometer pores at high molar strength enables capacitive deionization of saline water
Luca Cervini
DOI: 10.1039/C9SE00996E
Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cells
R. Vani, S. Ramaprabhu, Prathap Haridoss
DOI: 10.1039/C9SE01031A

![25553-77-9 - 2-[2-(哌嗪-1-基)-乙基]-1,3-二氧杂烷 25553-77-9 - 2-[2-(哌嗪-1-基)-乙基]-1,3-二氧杂烷](/structs/255/25553-77-9-5274.webp)