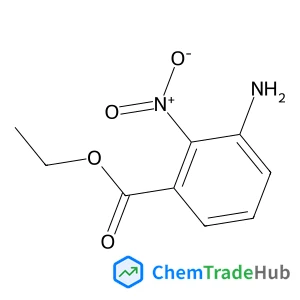
Ethyl 3-amino-2-nitrobenzoate(CAS号:193014-01-6)
3-氨基-2-硝基苯甲酸乙酯
基本信息
CAS号
193014-01-6
分子式
C9H10N2O4
分子量
210.19 g/mol
Quick Actions
基本物理性质
熔点
48-49 ºC
折射率
1.592
安全信息
查看安全信息危险说明
H315; H319; H335
同义词与参考文献
英文
- AKOS015919653
- Ethyl 3-amino-2-nitro-benzoate
- 3-amino-2-nitrobenzoic acid ethyl ester
- CS-W022668
- IJNYIIHVEXLJGI-UHFFFAOYSA-N
- ethyl3-amino-2-nitrobenzoate
- AS-18190
- Benzoic acid, 3-amino-2-nitro-, ethyl ester (9CI)
- MFCD12911920
- 193014-01-6
- SY110991
- Benzoic acid, 3-amino-2-nitro-, ethyl ester
- SCHEMBL3789796
- DTXSID40592737
- ethyl 3-amino-2-nitrobenzoate
- AMY7604
- J-520840
- SB78727
- A856767
- FT-0703730
- Ethyl 3-amino-2-nitrobenzoate
- Benzoic acid,3-amino-2-nitro-, ethyl ester
- 3-Amino-2-nitro-benzoesaeure-aethylester
- 3-amino-2-nitro-benzoic acid ethyl ester
- AGN-PC-01U7V3
- ANW-47590
- CTK8B5104
- RP26553
- SureCN3789796
- CM12433
- EN001903
- AX8208791
- W4131
- ST24030552
中文
- 3-氨基-2-硝基苯甲酸乙酯
MDL_Number
MFCD12911920
CAS号
193014-01-6
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 北京百灵威科技有限公司 中国 - 北京百灵威科技有限公司 |
|||||
 中国 - 上海阿拉丁生化科技股份有限公司 中国 - 上海阿拉丁生化科技股份有限公司 |
|||||
 中国 - 上海源叶生物科技有限公司 中国 - 上海源叶生物科技有限公司 |
|||||
 中国 - 湖北众善奉行医药科技有限公司 中国 - 湖北众善奉行医药科技有限公司 |
|||||
 中国 - 江苏瑞佳机电设备制有限公司 中国 - 江苏瑞佳机电设备制有限公司 |
|||||
 中国 - 武汉八颗星生物科技有限公司 中国 - 武汉八颗星生物科技有限公司 |
|||||
 德国 - 内部自动化有限公司 德国 - 内部自动化有限公司 |
|||||
 德国 - 岛津德国有限公司 德国 - 岛津德国有限公司 |
相关文献
Synthesis of aviation fuel from bio-derived isophorone
Courtney Ford Ryan, Cameron M. Moore, Juan H. Leal, Troy A. Semelsberger, Jenny K. Banh, Junqing Zhu, Charles S. McEnally, Lisa D. Pfefferle, Andrew D. Sutton
DOI: 10.1039/C9SE01014A
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
A new neodymium–phosphine compound for supercapacitors with long-term cycling stability
Xiaoyu Li, Huimin Chen, Chenyu Yang, Yafeng Li
DOI: 10.1039/D1CC00650A
Interfacial engineering of a polymer–MOF composite by in situ vitrification
Rijia Lin, Jingwei Hou, Mengran Li, Zhanke Wang, Lei Ge, Shichun Li, Zhonghua Zhu, Thomas D. Bennett, Vicki Chen
DOI: 10.1039/D0CC00664E
Catalytic depolymerization of Kraft lignin to produce liquid fuels via Ni–Sn metal oxide catalysts
Baikai Zhang, Wenzhi Li, Xiaomeng Dou, Jindong Wang, Lele Jin, Ajibola T. Ogunbiyi, Xiaosen Li
DOI: 10.1039/C9SE01089K
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E
Co9S8 integrated into nitrogen/sulfur dual-doped carbon nanofibers as an efficient oxygen bifunctional electrocatalyst for Zn–air batteries
Weiwei Zheng, Jiangquan Lv, Huabin Zhang, Hai-Xia Zhang, Jian Zhang
DOI: 10.1039/C9SE01130G
A model-based comparison of Ru and Ni catalysts for the Sabatier reaction
DOI: 10.1039/C9SE00787C
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D