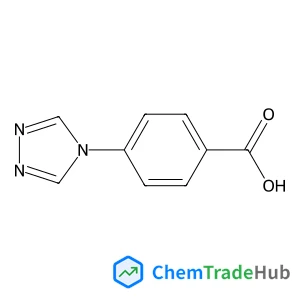
4-(4H-1,2,4-triazol-4-yl)benzoic acid(CAS号:157069-48-2)
聚(氧化乙烯),4-arm,端琥珀酰重胺基戊二酸
基本信息
CAS号
157069-48-2
分子式
C9H7N3O2
分子量
189.17 g/mol
Quick Actions
基本物理性质
安全信息
查看安全信息危险说明
H315-H319
危险类别
IRRITANT
同义词与参考文献
英文
- 4-(4H-1,2,4-Triazol-4-yl)benzoic acid
- 4-(1,2,4-triazol-4-yl)benzoic acid
- F2167-0140
- 4-(p-Carboxyphenyl)-1,2,4-triazole
- TIMTEC-BB SBB010789
- CHEMBRDG-BB 4016177
- 4-[1,2,4]TRIAZOL-4-YL-BENZOIC ACID
- 4-(4H-1,2,4-triazol-4-yl)benzoic acid(SALTDATA: FREE)
- Benzoic acid, 4-(4H-1,2,4-triazol-4-yl)-
- ZERO/005730
- QCNDLFQKKMPZKH-UHFFFAOYSA-N
- 4940AD
- BBL030566
- STK667344
- SBB010789
- 4-(4-carboxyphenyl)-1,2,4-triazole
- BAS 12820873
- 4-(4-Carboxyphenyl)-4H-1,2,4-triazole
- ST
- Hcpt
- AMY12178
- AKOS000112636
- SCHEMBL1664108
- A921341
- 4-(4H-1,2,4-triazol-4-yl)benzoic acid
- MFCD06654839
- T3788
- Z970090412
- 4-(4H-1,2,4-Triazol-4-yl)benzoic acid, AldrichCPR
- DTXSID80390355
- s12291
- EN300-62433
- 4-(4H-1,2,4-triazol-4-yl)benzoicacid
- AS-64508
- 4-[1,2,4]Triazol-4-yl-benzoic acid
- YSWG079
- CS-0110917
- 157069-48-2
- SY179927
中文
- 4-(4H-1,2,4-三氮唑-4-基)苯甲酸
- 4-[1,2,4]噻唑-4-苯甲酸
- 聚(氧化乙烯),4-arm,端琥珀酰重胺基戊二酸
- 4-(p-羧苯基)-1,2,4-三氮唑
- 4-[1,2,4]三氮唑-4-苯甲酸
MDL_Number
MFCD06654839
CAS号
157069-48-2
Customs_Code
2933990090
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 北京百灵威科技有限公司 中国 - 北京百灵威科技有限公司 |
|||||
 中国 - 合肥钼凯医药科技有限公司 中国 - 合肥钼凯医药科技有限公司 |
|||||
 中国 - 乐研试剂 中国 - 乐研试剂 |
|||||
 中国 - 河南阿尔法化工有限公司 中国 - 河南阿尔法化工有限公司 |
|||||
 中国 - 广东翁江化学试剂有限公司 中国 - 广东翁江化学试剂有限公司 |
|||||
 中国 - 金锦乐(湖南)化学有限公司 中国 - 金锦乐(湖南)化学有限公司 |
|||||
 中国 - 陕西晨曦之光化学科技有限公司 中国 - 陕西晨曦之光化学科技有限公司 |
|||||
 德国 - 劳工技术塔斯勒(LTT)有限公司 德国 - 劳工技术塔斯勒(LTT)有限公司 |
相关文献
High-performance tungsten carbide electrocatalysts for the hydrogen evolution reaction
Jing Li, Bao Wang, Wei Liu
DOI: 10.1039/C9SE00853E
Building microsphere–nanosheet structures in N-doped carbon to improve its performance in the oxygen reduction reaction and vanadium redox flow batteries
Baobing Huang, Yuchuan Liu, Miao Xia, Jiugen Qiu, Zailai Xie
DOI: 10.1039/C9SE00851A
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J
A hollow neuronal carbon skeleton with ultrahigh pyridinic N content as a self-supporting potassium-ion battery anode
Yongwen Sun, Ya Zhang, Zheng Xing, Denghu Wei, Quanchao Zhuang
DOI: 10.1039/C9SE00889F
A model-based comparison of Ru and Ni catalysts for the Sabatier reaction
DOI: 10.1039/C9SE00787C
Interfacial engineering of a polymer–MOF composite by in situ vitrification
Rijia Lin, Jingwei Hou, Mengran Li, Zhanke Wang, Lei Ge, Shichun Li, Zhonghua Zhu, Thomas D. Bennett, Vicki Chen
DOI: 10.1039/D0CC00664E
The limits to biocatalysis: pushing the envelope
Roger A. Sheldon, Dean Brady
DOI: 10.1039/C8CC02463D
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
Three-terminal III–V/Si tandem solar cells enabled by a transparent conductive adhesive
Manuel Schnabel, Michael Rienäcker, Emily L. Warren, Paul F. Ndione, Bill Nemeth, Talysa R. Klein, Maikel F. A. M. van Hest, John F. Geisz, Robby Peibst, Paul Stradins, Adele C. Tamboli
DOI: 10.1039/C9SE00893D


![25553-77-9 - 2-[2-(哌嗪-1-基)-乙基]-1,3-二氧杂烷 25553-77-9 - 2-[2-(哌嗪-1-基)-乙基]-1,3-二氧杂烷](/structs/255/25553-77-9-5274.webp)