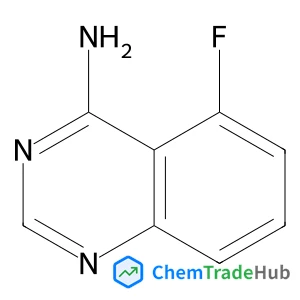
5-fluoroquinazolin-4-amine(CAS号:137553-48-1)
4-氨基-5-氟喹唑啉
基本信息
CAS号
137553-48-1
分子式
C8H6FN3
分子量
163.15 g/mol
Quick Actions
基本物理性质
熔点
212-214°C
安全信息
查看安全信息危险说明
Irritant
危险类别
IRRITANT
同义词与参考文献
英文
- 5-fluoro-4-quinazolinamine
- 4-Quinazolinamine, 5-fluoro-
- EN300-51566
- CS-0084745
- FS-1066
- DTXSID20351960
- A18186
- A807275
- 4-Quinazolinamine,5-fluoro-
- 4-Amino-5-fluoroquinazoline, AldrichCPR
- 5-fluoranylquinazolin-4-amine
- 5-fluoroquinazolin-4-amine
- 137553-48-1
- MFCD00153064
- SCHEMBL2124702
- D74557
- CCG-44808
- 4-Amino-5-Fluoroquinazoline
- AKOS005203452
- SR-01000634622-1
- FT-0617521
- KKLLTCMELJRTDI-UHFFFAOYSA-N
- HMS1668L14
- 5-Fluoroquinazolin-4-amine
- 4-Amino-5-fluoroquinazoline
- 5-fluoro-4-Quinazolinamine
中文
- 4-氨基-5-氟喹唑啉
- 4-氨基-5-氟奎唑啉
MDL_Number
MFCD00153064
CAS号
137553-48-1
Customs_Code
2933990090
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 湖北省德奥化研医药科技有限责任公司 中国 - 湖北省德奥化研医药科技有限责任公司 |
|||||
 中国 - 上海捷世凯生物科技有限公司 中国 - 上海捷世凯生物科技有限公司 |
|||||
 中国 - 乐研试剂 中国 - 乐研试剂 |
|||||
 德国 - NMI图宾根大学自然科学与医学研究所 德国 - NMI图宾根大学自然科学与医学研究所 |
|||||
 法国 - Cristal France SAS 法国 - Cristal France SAS |
|||||
 中国 - 南京定达医药科技有限公司 中国 - 南京定达医药科技有限公司 |
|||||
 中国 - 南京奥德赛化工有限公司 中国 - 南京奥德赛化工有限公司 |
|||||
 中国 - 高邮市光明化工厂 中国 - 高邮市光明化工厂 |
期刊推荐

Advances in Colloid and Interface Science

Journal of the Chinese Chemical Society

Corrosion Science

Chemistry of Heterocyclic Compounds

Doklady Chemistry

Journal of the American Chemical Society

Chemistry of Natural Compounds

Australian Journal of Chemistry

Accounts of Chemical Research

Bulletin of the Chemical Society of Japan
相关文献
Transition metal chemistry in synthetically viable alkaline earth complexes M(Cp)3− (M = Ca, Sr, Ba)
Bin Huo, Rui Sun, Bo Jin, Lingfei Hu, Jian-Hong Bian, Xiao-Ling Guan, Caixia Yuan, Gang Lu, Yan-Bo Wu
DOI: 10.1039/D1CC01753E
Pulsed laser rusted stainless steel: a robust electrode material applied for energy storage and generation applications
Namachivayam Karthik, Tian Tian, Thomas Nesakumar Jebakumar Immanuel Edison, Raji Atchudan, Yong Rok Lee, Seongbeom Kim, Dangsheng Xiong
DOI: 10.1039/C9SE00676A
Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA delivery
Marco Herrera, Jeonghwan Kim, Yulia Eygeris, Antony Jozic
DOI: 10.1039/D0BM01947J
Engineering nanoporous organic frameworks to stabilize naked Au clusters: a charge modulation approach
Chengcheng Tian, Xiang Zhu, Huize Wang, Hai Wang, Carter W. Abney, Ning Zhang
DOI: 10.1039/C8CC02966K
Strong circularly polarized luminescence of an octahedral chromium(iii) complex
Carolin Dee, Francesco Zinna, Winald R. Kitzmann, Gennaro Pescitelli, Katja Heinze, Lorenzo Di Bari, Michael Seitz
DOI: 10.1039/C9CC06909G
Developing a novel high performance NaNbO3-based lead-free dielectric capacitor for energy storage applications
DOI: 10.1039/C9SE00836E
Nickel-containing N-doped carbon as effective electrocatalysts for the reduction of CO2 to CO in a continuous-flow electrolyzer
Bert De Mot, Daniel Choukroun, Chen Li, Annick Hubin, Sara Bals, Jonas Hereijgers
DOI: 10.1039/C9SE00814D
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
High-performance tungsten carbide electrocatalysts for the hydrogen evolution reaction
Jing Li, Bao Wang, Wei Liu
DOI: 10.1039/C9SE00853E
An overview of latest advances in exploring bioactive peptide hydrogels for neural tissue engineering
Pooja Sharma, Vijay Kumar Pal, Sangita Roy
DOI: 10.1039/D0BM02049D