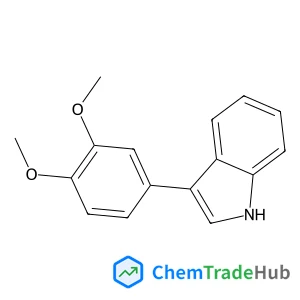
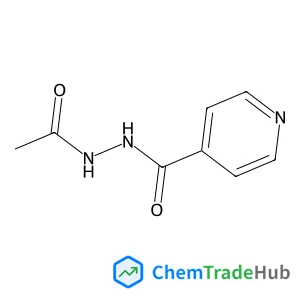
Acetyl Isoniazid(CAS号:1078-38-2)
乙酰异烟肼
基本信息
CAS号
1078-38-2
分子式
C8H9N3O2
分子量
179.18 g/mol
Quick Actions
基本物理性质
熔点
158-159°C
沸点
311.69°C (rough estimate)
折射率
1.7400 (estimate)
安全信息
查看安全信息同义词与参考文献
英文
- (N)1-Acetylisoniazid
- 2-Acetylhydrazide-4-pyridinecarboxylic Acid
- J42942UVUN
- Acetyl isoniazid
- NCGC00245164-01
- 4-22-00-00606 (Beilstein Handbook Reference)
- 1-Acetyl-2-isonicotinoylhydrazine
- 1078-38-2
- SMR000116629
- N-Isonicotinoyl-N'-acetyl-hydrazin
- CBDivE_005562
- CHEMBL1442231
- N-acetyl-N'-isonicotinoylhydrazine
- 4-Pyridinecarboxylicacid, 2-acetylhydrazide
- N'-acetylpyridine-4-carbohydrazide
- DTXSID1020013
- HMS2469P12
- UNII-J42942UVUN
- 4-Ethylcatechol 100 microg/mL in Acetonitrile
- 1-Acetyl-2-isonicotinoyl-hydrazine
- SCHEMBL1415953
- C07585
- BRN 0154108
- 4-Pyridinecarboxylic acid, 2-acetylhydrazide
- N-Acetylisonicotinylhydrazide
- CHEBI:7207
- Acetylisoniazid
- J-002022
- SR-01000389726-1
- AKOS001586427
- SR-01000389726
- BS-25514
- N'-acetylisoniazid
- Q27107456
- Acetylisoniazide;N-Monoacetylisoniazid; NSC 36084
- Isonicotinic acid N'-acetyl-hydrazide
- N'-Acetylisonicotinohydrazide
- N-Acetylisoniazid
- N-[(pyridin-4-yl)carbonyl]ethanehydrazonic acid
- FT-0661273
- MLS000526155
- NS00115966
- Hydrazine, 1-acetyl-2-isonicotinoyl-
- Acetyl Isoniazid
- (N)1-acetylisoniazid
- acetylisonicotinoylhydrazine
- N-Acetyl-Isoniazid
- N-Acetyl-N'-isonicotinoyl-hydrazin
- N-Acetyl-N'-isonicotinsaeure-hydrazin
- NSC-36084
- Acetylisoniazide
- N-Monoacetylisoniazid
- N-Acetyl-N'-isonicotinoylhydrazine
- HYDRAZINE,1-ACETYL-2-ISONICOTINYL-
- ACETYLISONIAZID
- N'-acetylisonicotinohydrazide
中文
- 4-吡啶羧酸2-乙酰基酰肼
- 5 - 乙炔基H-咪唑并[1,2-a]吡啶
- 乙酰异烟肼
MDL_Number
MFCD00466330
CAS号
1078-38-2
Customs_Code
2933399090
供应商信息
| 供应商名称 | 会员等级 | 认证状态 | 主要类别 | 最小订购量 | 操作 |
|---|---|---|---|---|---|
 中国 - 上海捷世凯生物科技有限公司 中国 - 上海捷世凯生物科技有限公司 |
|||||
 中国 - 上海柯维化学技术有限公司 中国 - 上海柯维化学技术有限公司 |
|||||
 中国 - 上海赛可锐生物科技有限公司 中国 - 上海赛可锐生物科技有限公司 |
|||||
 中国 - 上海瀚思化工有限公司 中国 - 上海瀚思化工有限公司 |
|||||
 中国 - 上海源叶生物科技有限公司 中国 - 上海源叶生物科技有限公司 |
|||||
 中国 - 佛山市诺银贵金属材料有限公司 中国 - 佛山市诺银贵金属材料有限公司 |
|||||
 瑞士 - Vitaris AG 瑞士 - Vitaris AG |
|||||
 中国 - 广州恒星冷冻机械制造有限公司 中国 - 广州恒星冷冻机械制造有限公司 |
相关文献
Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)
DOI: 10.1039/C7CC09520A
Novel aqueous amine looping approach for the direct capture, conversion and storage of CO2 to produce magnesium carbonate
Meishen Liu, Hassnain Asgar, Soenke Seifert, Greeshma Gadikota
DOI: 10.1039/C9SE00316A
Direct arylation polycondensation towards water/alcohol-soluble conjugated polymers as the electron transporting layers for organic solar cells
Qingwu Yin, Zhenfeng Wang, Boming Xie, Fei Huang, Yong Cao
DOI: 10.1039/D1CC01128F
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E
Heterogeneous toroidal spiral particles for islet encapsulation
Paola Leon Plata, Maryam Zaroudi, Chun-Yin Lee, Colin Foster, Ludwig C. Nitsche, Peter D. Rios, Yong Wang, Jose Oberholzer
DOI: 10.1039/D0BM02082F
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
Chemoproteomics-based target profiling of sinomenine reveals multiple protein regulators of inflammation
Lianguo Chen, Hong-jian Wang, Teng-fei Ji, Chong-Jing Zhang
DOI: 10.1039/D1CC01522B
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
Three-terminal III–V/Si tandem solar cells enabled by a transparent conductive adhesive
Manuel Schnabel, Michael Rienäcker, Emily L. Warren, Paul F. Ndione, Bill Nemeth, Talysa R. Klein, Maikel F. A. M. van Hest, John F. Geisz, Robby Peibst, Paul Stradins, Adele C. Tamboli
DOI: 10.1039/C9SE00893D

![224-53-3 - 二苯并[C,H]吖啶 224-53-3 - 二苯并[C,H]吖啶](/structs/224/224-53-3-97c9.webp)