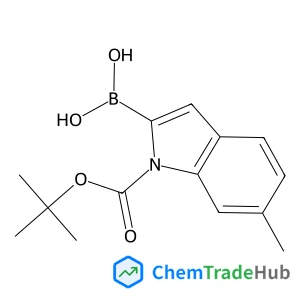
1-BOC-6-methylindole-2-boronic acid(CAS番号:850568-51-3)
1-BOC-6-メチルインドール-2-ボロニルกลคชีวสาร
基本情報
CAS番号
850568-51-3
分子式
C14H18BNO4
分子量
275.11 g/mol
Quick Actions
基本的な物理特性
融点
102-104
沸点
456.8±55.0 °C(Predicted)
密度
1.16±0.1 g/cm3(Predicted)
引火点
230.1°C
屈折率
1.541
安全情報
安全情報を表示危険性の表示
Irritant/Keep Cold
同義語と参考文献
英語
- 1-T-BUTOXYCARBONYL-6-METHYLINDOLE-2-BORONIC ACID
- 1-BOC-6-METHYL-2-INDOLEBORONIC ACID
- 1-BOC-6-METHYLINDOLE-2-BORONIC ACID
- 6-METHYLINDOLE-2-BORONIC ACID, BOC PROTECTED
- 6-Methylindole-2-boronic acid, N-BOC protected
- 6-Methylindole-2-boronic acid, N-BOC protected 95%
- 6-Methyl-1H-indole-2-boronic acid, N-BOC protected
- 1H-Indole-1-carboxylic acid, 2-borono-6-methyl-, 1-(1,1-dimethylethyl) ester
- (1-(tert-Butoxycarbonyl)-6-methyl-1H-indol-2-yl)boronic acid
- 1-Boc-6-Methylindole-2-Boronic Acid
- [6-methyl-1-[(2-methylpropan-2-yl)oxycarbonyl]indol-2-yl]boronic acid
- {1-[(tert-butoxy)carbonyl]-6-methyl-1H-indol-2-yl}boronic acid
- 1H-Indole-1-carboxylicacid, 2-borono-6-methyl-, 1-(1,1-dimethylethyl) ester
- KLHBTAWDTXMJLT-UHFFFAOYSA-N
- N-Boc-6-methyl-indoleboronic acid
- VB10502
- AB21402
- (1-(tert-butoxycarbonyl)-6-methyl-1H-indol-2-yl)boronic acid
- SCHEMBL594086
- AKOS015836493
- CS-0002252
- 1-Boc-6-methylindole-2-boronic acid, AldrichCPR
- W17386
- DB-019046
- [6-methyl-1-[(2-methylpropan-2-yl)oxycarbonyl]indol-2-yl]boronic Acid
- (1-(tert-Butoxycarbonyl)-6-methyl-1H-indol-2-yl)boronicacid
- 1-BOC-6-methylindole-2-boronic acid
- AS-70950
- 850568-51-3
- 1-(tert-butoxycarbonyl)-6-methyl-1H-indol-2-ylboronic acid
- DTXSID70393431
- MFCD04973738
MDL_Number
MFCD04973738
CAS番号
850568-51-3
Customs_Code
2933299090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Beijing Bailingwei Technology Co., Ltd. 中国 - Beijing Bailingwei Technology Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Shanghai Jixiang Biotechnology Co., Ltd. 中国 - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Tianjin Bram Technology Co., Ltd. 中国 - Tianjin Bram Technology Co., Ltd. |
|||||
 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. |
|||||
 ドイツ - INTAS Science Imaging Instruments GmbH ドイツ - INTAS Science Imaging Instruments GmbH |
|||||
 中国 - Shanxi De Zhi Bei Metal Materials Co., Ltd. 中国 - Shanxi De Zhi Bei Metal Materials Co., Ltd. |
関連論文
Carbon-based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels
Mufeedah Muringa Kandy
DOI: 10.1039/C9SE00827F
Facile room-temperature growth of nanostructured CuBi2O4 for selective electrochemical reforming and photoelectrochemical hydrogen evolution reactions
Chia-Yu Lin, Shao-Yu Lin, Ming-Chun Tsai, Cheng-Hsien Wu
DOI: 10.1039/C9SE00558G
Carbon and carbon composites obtained using deep eutectic solvents and aqueous dilutions thereof
Gaspar Carrasco-Huertas, Rafael J. Jiménez-Riobóo, María Concepción Gutiérrez, María Luisa Ferrer, Francisco del Monte
DOI: 10.1039/D0CC00681E
PEST (political, environmental, social & technical) analysis of the development of the waste-to-energy anaerobic digestion industry in China as a representative for developing countries
Habiba Khalid, Hongyan Zhang, Caiyan Liu, Wei Li, Muhammad Khubaib Abuzar, Farrukh Raza Amin, Guangqing Liu, Chang Chen
DOI: 10.1039/C9SE00692C
Redox responsive Pluronic micelle mediated delivery of functional siRNA: a modular nano-assembly for targeted delivery
Sandeep Kadekar, Ganesh N. Nawale, Vadim Le Joncour, Pirjo Laakkonen, Jöns Hilborn, Oommen P. Varghese, Oommen P. Oommen
DOI: 10.1039/D1BM00428J
Electrospun hydrogels for dynamic culture systems: advantages, progress, and opportunities
M. Gregory Grewal
DOI: 10.1039/D0BM01588A
Biomaterials Science Emerging Investigators 2021
Maria E. Southall
DOI: 10.1039/D1BM90053F
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
Insights into the mechanism of photosynthetic H2 evolution catalyzed by a heptacoordinate cobalt complex
Fiorella Lucarini, Jennifer Fize, Adina Morozan, Mirco Natali, Mariachiara Pastore, Vincent Artero, Albert Ruggi
DOI: 10.1039/C9SE00434C