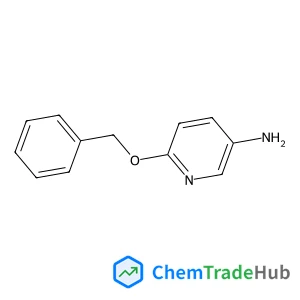
6-(Benzyloxy)-3-pyridinamine(CAS番号:75926-65-7)
基本情報
CAS番号
75926-65-7
分子式
C12H12N2O
分子量
200.24 g/mol
Quick Actions
基本的な物理特性
安全情報
安全情報を表示同義語と参考文献
英語
- AKOS000137375
- FT-0734763
- US18128000
- 6-[(Phenylmethyl)oxy]-3-pyridinamine
- 5-Amino-2-benzyloxypyridine
- NIOSH/US1812800
- TS-02837
- 6-benzyloxy-pyridin-3-ylamine
- EN300-45483
- 6-benzyloxypyridin-3-amine
- 6-(Benzyloxy)-3-pyridinamine #
- DTXSID10340287
- 75926-65-7
- CS-0021987
- 6-(benzyloxy)pyridin-3-amine
- SCHEMBL224083
- F74416
- SB78380
- Pyridine, 5-amino-2-(benzyloxy)-
- 6-(benzyloxy)pyridin-3-amine, AldrichCPR
- 6-Benzyloxypyridin-3-ylamine
- 6-phenylmethoxypyridin-3-amine
- MFCD01692494
- SY174142
- 3-Pyridinamine, 6-(phenylmethoxy)-
- 5-Amino-2-(benzyloxy)pyridine
- 6-(Benzyloxy)pyridin-3-amine
- 3-amino-6-(benzyloxy)pyridine
- 6-(Benzyloxy)-3-pyridinamine
- 6-[(phenylmethyl)oxy]-3-pyridinamine
- 6-Benzyloxy-[3]pyridylamin
- 6-benzyloxy-[3]pyridylamine
- AC1Q52I4
- SBB051835
- SureCN224083
MDL_Number
MFCD01692494
CAS番号
75926-65-7
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Tengzhun BioScience Co., Ltd. 中国 - Shanghai Tengzhun BioScience Co., Ltd. |
|||||
 中国 - Hubei Hanwei Chemical Co., Ltd. 中国 - Hubei Hanwei Chemical Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Sichuan Befeng Biotechnology Co., Ltd. 中国 - Sichuan Befeng Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 フランス - Michel Baulé SA フランス - Michel Baulé SA |
|||||
 日本 - Mitsubishi Chemical Corporation 日本 - Mitsubishi Chemical Corporation |
関連論文
Vapor-fed photoelectrolysis of water at 0.3 V using gas-diffusion photoanodes of SrTiO3 layers
Hyosuke Mukohara, Hiroki Sato, Chihiro Tateishi, Hiromasa Sato
DOI: 10.1039/C9SE01068H
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Increasing efficiency of perovskite solar cells using low concentrating photovoltaic systems
Hasan Baig, Hiroyuki Kanda, Abdullah M. Asiri, Mohammad Khaja Nazeeruddin, Tapas Mallick
DOI: 10.1039/C9SE00550A
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosity
An-Hua Liu, Jie-Jie Li, Bai-Hao Ren, Xin-Ru Sha, He Jiang, Xiao-Bing Lu
DOI: 10.1039/C9SE00756C
Direct arylation polycondensation towards water/alcohol-soluble conjugated polymers as the electron transporting layers for organic solar cells
Qingwu Yin, Zhenfeng Wang, Boming Xie, Fei Huang, Yong Cao
DOI: 10.1039/D1CC01128F
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
Boronic acid liposomes for cellular delivery and content release driven by carbohydrate binding‡
Xiaoyu Zhang, Daiane S. Alves, Jinchao Lou, Shelby D. Hill, Francisco N. Barrera, Michael D. Best
DOI: 10.1039/C8CC00820E
High-performance tungsten carbide electrocatalysts for the hydrogen evolution reaction
Jing Li, Bao Wang, Wei Liu
DOI: 10.1039/C9SE00853E
Sugar ketals as a platform molecule to overcome the limitation of converting biomass into green-hydrocarbons in a typical refinery
Matheus Souza, Joana Pinto, Laura M. Esteves, Yiu Lau Lam, Leandro Soter de Mariz e Miranda
DOI: 10.1039/C9SE00379G
Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloaddition
Renzeng Chen, Danbo Wang, Wenbo Hao, Feng Shao, Yingjie Zhao
DOI: 10.1039/D1CC02179F

![37845-14-0 - 4,5-Dihydronaphtho[1,2-c]furan-1,3-dione 37845-14-0 - 4,5-Dihydronaphtho[1,2-c]furan-1,3-dione](/structs/378/37845-14-0-f8d0.webp)
![57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone 57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone](/structs/574/57423-71-9-78dc.webp)
![503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene 503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene](/structs/503/503070-57-3-bc25.webp)
![25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine 25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine](/structs/255/25553-77-9-5274.webp)









