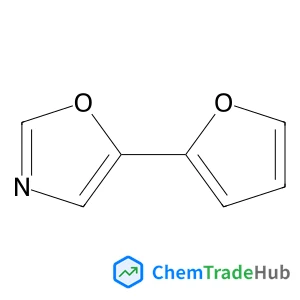
5-(2-Furyl)-1,3-oxazole(CAS番号:70380-67-5)
基本情報
CAS番号
70380-67-5
分子式
C7H5NO2
分子量
135.12 g/mol
Quick Actions
基本的な物理特性
沸点
102 °C
密度
1.19
引火点
96.5°C
屈折率
1.502
安全情報
安全情報を表示危険性の表示
Irritant
同義語と参考文献
英語
- SCHEMBL3185621
- 5-(Furan-2-yl)oxazole
- 5-(furan-2-yl)-1,3-oxazole
- 5-(2-Furyl)-1,3-oxazole
- LYPXTVWBKURKQH-UHFFFAOYSA-N
- AKOS015993853
- FT-0619584
- 9X-0867
- 5-FUR-2-YL-1,3-OXAZOLE
- oxazole, 5-(2-furanyl)-
- DTXSID70353191
- CS-0360610
- MFCD00085092
- 70380-67-5
- 5-furan-2-yl-oxazole
- Oxazole, 5-(2-furanyl)-
- 5-(2-furanyl)Oxazole
- 5-(2-FURYL)-1,3-OXAZOLE
- 5-(FUR-2-YL)-1,3-OXAZOLE
CAS番号
70380-67-5
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Fuzhou Tianze Bio Pharmaceutical Technology Co., Ltd. 中国 - Fuzhou Tianze Bio Pharmaceutical Technology Co., Ltd. |
|||||
 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Shanghai Jixiang Biotechnology Co., Ltd. 中国 - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. |
|||||
 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 イタリア - Brembana Costruzioni Industriali s.r.l. イタリア - Brembana Costruzioni Industriali s.r.l. |
|||||
 ドイツ - Gestra AG ドイツ - Gestra AG |
関連論文
Photoactivatable fluorophores for durable labelling of individual cells
Hiroki Kashima, Mako Kamiya, Shotaro Nakano, Masayuki Miura
DOI: 10.1039/D1CC01488A
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Kan Zhang, Ming-Ming Yang, Shan Xu, Hua-Ming Sun, Jin-Lei Zhang, Zi-Wei Gao, Wei-Qiang Zhang
DOI: 10.1039/C8CC00324F
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalysts
Minling Fang, Zhiping Zheng, Jiayu Chen, Qian Chen, Deyu Liu, Binbin Xu, Jianyang Wu, Qin Kuang
DOI: 10.1039/C9SE00678H
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structure
Li-Ping Cheng, Zhi Wang, Qiao-Yu Wu, Hai-Feng Su, Tao Peng, Geng-Geng Luo, Yan-An Li, Di Sun, Lan-Sun Zheng
DOI: 10.1039/C8CC00014J
An environmentally friendly natural polymer as a universal interfacial modifier for fullerene and non-fullerene polymer solar cells
Xiaojing Wang, Shuwang Yi, Zhicai He, Xinhua Ouyang, Hong-Bin Wu, Weiguo Zhu, Bin Zhang, Yong Cao
DOI: 10.1039/C9SE01079C
An overview of latest advances in exploring bioactive peptide hydrogels for neural tissue engineering
Pooja Sharma, Vijay Kumar Pal, Sangita Roy
DOI: 10.1039/D0BM02049D
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
Carbon-based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels
Mufeedah Muringa Kandy
DOI: 10.1039/C9SE00827F
Nickel-containing N-doped carbon as effective electrocatalysts for the reduction of CO2 to CO in a continuous-flow electrolyzer
Bert De Mot, Daniel Choukroun, Chen Li, Annick Hubin, Sara Bals, Jonas Hereijgers
DOI: 10.1039/C9SE00814D



![700874-71-1 - 7-[2-(4-Morpholinyl)ethoxy]-4-[2-(2-pyridinyl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline 700874-71-1 - 7-[2-(4-Morpholinyl)ethoxy]-4-[2-(2-pyridinyl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline](/structs/700/700874-71-1-fbbc.webp)