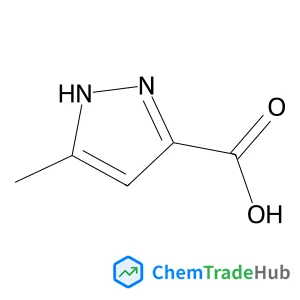
5-Methyl-1H-pyrazole-3-carboxylic acid(CAS番号:696-22-0)
基本情報
CAS番号
696-22-0
分子式
C5H6N2O2
分子量
126.11 g/mol
Quick Actions
基本的な物理特性
融点
236-240 ºC
安全情報
安全情報を表示同義語と参考文献
英語
- AKOS000265556
- CHEMBL391574
- HY-33009
- AM804242
- 3-methyl-1H-pyrazole-5-carboxylic acid
- PYRAZOLE-3(OR 5)-CARBOXYLIC ACID, 5(OR 3)-METHYL-
- CHEBI:74739
- A6735
- 4-25-00-00731 (Beilstein Handbook Reference)
- PB13019
- J-512825
- CCG-266102
- U71778T48T
- SDCCGMLS-0065489.P001
- 3-Methylpyrazole-5-carboxylic acid, 97%
- 5-Methyl-2H-pyrazole-3-carboxylic acid
- AS-5392
- MFCD00090754
- 3-METHYL-1H-PYRAZOLE-5-CARBOXYLICACID
- M2607
- AS-057278
- AS 057278
- AM20100068
- s5434
- AB00672916-03
- LITHIUMTANTALATE
- BB 0219106
- Pyrazole-5-carboxylic acid, 3-methyl-
- 5-METHYLPYRAZOLE-3-CARBOXYLIC ACID [MI]
- 1H-PYRAZOLE-3-CARBOXYLIC ACID, 5-METHYL-
- 5-methyl-pyrazole-3-carboxylic acid
- 5-Methyl-1H-pyrazole-3-carboxylic acid #
- Z752370868
- UNII-U71778T48T
- Pyrazole-3-carboxylic acid, 5-methyl-
- 5-Methyl-1H-pyrazole-3-carboxylic acid, AldrichCPR
- 1H-PYRAZOLE-5-CARBOXYLIC ACID, 3-METHYL-
- 5-methylpyrazole-3-carboxylicacid
- 5-Methyl-1H-pyrazole-3-carboxylic acid
- BB 0253991
- AB00672916-01
- AKOS000145258
- 5(OR 3)-METHYL-PYRAZOLE-3(OR 5)-CARBOXYLIC ACID
- DTXSID50193198
- SB19897
- NSC-1408
- BDBM50211362
- 696-22-0
- F0917-7550
- AC-23127
- BP-10275
- 1H-Pyrazole-3-carboxylicacid, 5-methyl-
- FT-0601814
- 402-61-9
- 5-carb-oxy-3-methylpyrazole
- MFCD00462235
- AS057278
- SY007408
- BRN 0002906
- NCGC00326251-01
- CS-0166799
- EN300-41425
- 3-methyl-5-pyrazolecarboxylicacid
- NSC1408
- F2120-0002
- AKOS025394813
- 3-Methyl-5-Pyrazolecarboxylic Acid
- CL3431
- 5-METHYLPYRAZOLE-3-CARBOXYLIC ACID
- A9209
- 5-methyl-3-carboxyl-pyrazole
- FT-0601812
- SCHEMBL220072
- J-517731
- CS-D1083
- U 19425
- 1Y-0803
- Q27144868
- 3-Methylpyrazole-5-carboxylic acid
- 3-Methyl-1H-pyrazole-5-carboxylic acid
- 3-methyl-1H-pyrazole-5-carboxylic acid(SALTDATA: FREE)
- 3-methyl-5-carboxy-1H-pyrazole
- 3-methyl-5-pyrazolecarboxylic acid
- 3-methylpyrazole-5-carboxylic acid
- 3-methylpyrazolo-5-carboxylic acid
- 5-methyl-1(2)H-pyrazole-3-carboxylic acid
- 5-methyl-2H-pyrazole-3-carboxylic acid
- 5-Methylpyrazole-3-carboxylic acid
- WSMQKESQZFQMFW-UHFFFAOYSA-N
- InChI=1/C5H6N2O2/c1-3-2-4(5(8)9)
Beilstein_Registry_Number
0002906
MDL_Number
MFCD00462235
CAS番号
696-22-0
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Titan Shareholding Technology Co., Ltd. 中国 - Shanghai Titan Shareholding Technology Co., Ltd. |
|||||
 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Jixiang Biotechnology Co., Ltd. 中国 - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. |
|||||
 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 ドイツ - Zefa-Laborservice GmbH ドイツ - Zefa-Laborservice GmbH |
|||||
 ドイツ - heatsystems GmbH & Co. KG ドイツ - heatsystems GmbH & Co. KG |
関連論文
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
Ultra-thin NiFeSe nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting
Yu-Yang Sun, Mei-Yan Jiang, Guang-Ya Hou, Yi-Ping Tang, Min Liu
DOI: 10.1039/C9SE00905A
Microscopic insights into long-range 1D ordering in a dense semi-disordered molecular overlayer
Ryan T. Hannagan, Isaac Onyango, Amanda Larson, E. Charles H. Sykes
DOI: 10.1039/D1CC01574E
Effective utilisation of waste cooking oil in a single-cylinder diesel engine using alumina nanoparticles
Sumit Roy, Pranay Kumar Parsi, R. Sreeram Kotha, Sanmitra Barman, Kalluri Vinayak, Mili Mitra Roy, Rahul Banerjee
DOI: 10.1039/C9SE00393B
Boronic acid liposomes for cellular delivery and content release driven by carbohydrate binding‡
Xiaoyu Zhang, Daiane S. Alves, Jinchao Lou, Shelby D. Hill, Francisco N. Barrera, Michael D. Best
DOI: 10.1039/C8CC00820E
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
Synthesis of aviation fuel from bio-derived isophorone
Courtney Ford Ryan, Cameron M. Moore, Juan H. Leal, Troy A. Semelsberger, Jenny K. Banh, Junqing Zhu, Charles S. McEnally, Lisa D. Pfefferle, Andrew D. Sutton
DOI: 10.1039/C9SE01014A
Near infrared light activation of an injectable whole-cell cancer vaccine for cancer immunoprophylaxis and immunotherapy
Fei Wang, Junbin Gao, Shuanghu Wang, Jiamiao Jiang, Yicheng Ye, Juanfeng Ou, Shuwen Liu, Fei Peng, Yingfeng Tu
DOI: 10.1039/D1BM00542A
From zinco(ii) arsaketenes to silylene-stabilised zinco arsinidene complexes
Ernesto Ballestero-Martínez, Terrance J. Hadlington, Tibor Szilvási, Shenglai Yao, Matthias Driess
DOI: 10.1039/C8CC01928B

![503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene 503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene](/structs/503/503070-57-3-bc25.webp)
![57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone 57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone](/structs/574/57423-71-9-78dc.webp)

![56843-76-6 - 2-phenylthieno[2,3-d]pyrimidin-4-ol 56843-76-6 - 2-phenylthieno[2,3-d]pyrimidin-4-ol](/structs/568/56843-76-6-0035.webp)