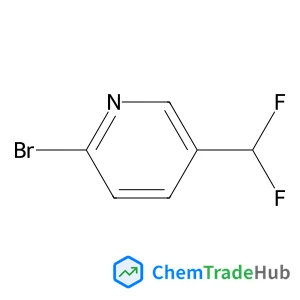
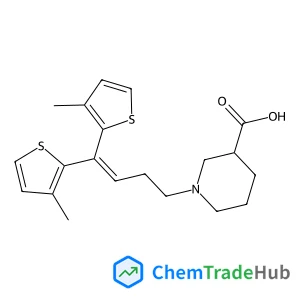
Dimethyl (1E,1'E)-N,N'-{sulfanediylbis[(methylcarbamoyl)oxy]}diethanimidothioate(CAS番号:59669-26-0)
基本情報
![Dimethyl (1E,1'E)-N,N'-{sulfanediylbis[(methylcarbamoyl)oxy]}diethanimidothioate Dimethyl (1E,1'E)-N,N'-{sulfanediylbis[(methylcarbamoyl)oxy]}diethanimidothioate](/structs/596/59669-26-0-4691.webp)
CAS番号
59669-26-0
分子式
C10H18N4O4S3
分子量
354.48 g/mol
Quick Actions
基本的な物理特性
融点
168-172 ºC
沸点
433.8±28.0 °C at 760 mmHg
密度
1.4000
引火点
216.2±24.0 °C
屈折率
1.6000 (estimate)
蒸気圧
0.0±1.0 mmHg at 25°C
安全情報
安全情報を表示危険性の表示
H301,H330,H400
危険物区分
6.1(b)
感度
对光敏感
毒性
LD50 in rats (mg/kg): 160 orally, >1600 dermally (Sousa)
同義語と参考文献
英語
- Larvin
- Thiodicarb
- bis[1-methylthioacetaldehyde-o-(n-methylcarbamoyl)oximino]sulfide
- dimethyl n,n'-[thiobis[(methylimino)carbonyloxy]]bis(thioimidoacetate)
- n,n'-[thiobis[(methylimino)carbonyloxy]]bis-ethanimidothioic acid dimethyl ester
- (3EZ,12EZ)-3,7,9,13-tetramethyl-5,11-dioxa-2,8,14-trithia-4,7,9,12-tetraazapentadeca-3,12-diene-6,10-dione
- Thiodicarb solution
- CHIPCO
- dimethyl N,N’-[thiobis[(methylimino)carbonyloxy]]bis[ethanimidothioate]
- lepicron
- NIVRAL
- semevin
- SKIPPER
- N,N'-[Thiobis[(methylimino)carbonyloxy]]bis-ethanimidothioic
- Bis[1-methylthioacetaldehyde-O-(N-methylcarbamoyl)oximino]sulfide
- Dimethyl N,N'-[thiobis[(methylimino)carbonyloxy]]bis(thioimidoacetate)
- N,N'-[Thiobis[(methylimino)carbonyloxy]]bis-ethanimidothioic acid dimethyl ester
MDL_Number
MFCD00145401
CAS番号
59669-26-0
Merck_Index
13,9403
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 中国 - Ningbo Sinochem Chemicals Co., Ltd. 中国 - Ningbo Sinochem Chemicals Co., Ltd. |
|||||
 中国 - Nantong Shi Zhuang Chemical Co., Ltd. 中国 - Nantong Shi Zhuang Chemical Co., Ltd. |
|||||
 中国 - Nanjing Renxin Chemical Co., Ltd. 中国 - Nanjing Renxin Chemical Co., Ltd. |
|||||
 中国 - Taixing Chemical Co., Ltd. 中国 - Taixing Chemical Co., Ltd. |
|||||
 中国 - Jiangsu Wanqi Biological Technology Co., Ltd. 中国 - Jiangsu Wanqi Biological Technology Co., Ltd. |
|||||
 ドイツ - Ludwig Melosch Vertriebs-GmbH & Co. ドイツ - Ludwig Melosch Vertriebs-GmbH & Co. |
|||||
 ドイツ - Steinhaus GmbH ドイツ - Steinhaus GmbH |
関連論文
An overview of latest advances in exploring bioactive peptide hydrogels for neural tissue engineering
Pooja Sharma, Vijay Kumar Pal, Sangita Roy
DOI: 10.1039/D0BM02049D
Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalks
Xiaobo Zhu, Shang-wen Tang, Wenyue Zhao, Xianliang Li, Zhengyi Lv, Li Yu
DOI: 10.1039/C9SE00906J
Synthesis of aviation fuel from bio-derived isophorone
Courtney Ford Ryan, Cameron M. Moore, Juan H. Leal, Troy A. Semelsberger, Jenny K. Banh, Junqing Zhu, Charles S. McEnally, Lisa D. Pfefferle, Andrew D. Sutton
DOI: 10.1039/C9SE01014A
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E
Ultra-thin NiFeSe nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting
Yu-Yang Sun, Mei-Yan Jiang, Guang-Ya Hou, Yi-Ping Tang, Min Liu
DOI: 10.1039/C9SE00905A
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structure
Li-Ping Cheng, Zhi Wang, Qiao-Yu Wu, Hai-Feng Su, Tao Peng, Geng-Geng Luo, Yan-An Li, Di Sun, Lan-Sun Zheng
DOI: 10.1039/C8CC00014J
Direct arylation polycondensation towards water/alcohol-soluble conjugated polymers as the electron transporting layers for organic solar cells
Qingwu Yin, Zhenfeng Wang, Boming Xie, Fei Huang, Yong Cao
DOI: 10.1039/D1CC01128F
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A