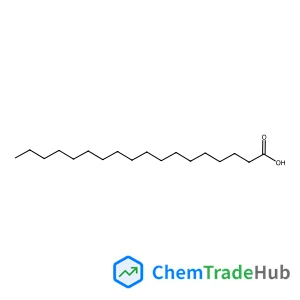
octadecanoic acid(CAS番号:57-11-4)
十八酸
基本情報
CAS番号
57-11-4
分子式
C18H36O2
分子量
284.48 g/mol
Quick Actions
基本的な物理特性
物理状態
Powder
融点
68.0 to 71.0 deg-C
沸点
361 °C(lit.)
水溶性
0.1-1 g/100 mL at 23 ºC
溶解度
1 to 10 mg/mL at 73° F (NTP, 1992)
屈折率
1.4299
蒸気圧
1 mmHg ( 173.7 °C)
蒸気密度
9.8 (NTP, 1992) (Relative to Air)
臭い
Slight odor suggesting tallow
分類と用途
用途
Widely used as a low-temperature plastic plasticizer, stabilizer, surfactant, demolding agent, and rubber vulcanization promoter. For water hardness determination, it is also used as an activator and diffusant for acidification. Used in the preparation of soap, cosmetics, pharmaceuticals, and other organic chemicals. Used as an emulsifier for oil-based drilling fluids. Also used as a lubricant and wetting agent in rubber industry and textile industry. Lubricant; anti-caking agent; gum base; defoamer and other food additives (such as glycerol stearate, sorbitan stearate ester, sucrose ester, etc.) as raw materials. Used as a vulcanization promoter for natural rubber, synthetic rubber (except butyl rubber) and latex, and as raw materials for plastic plasticizers and stabilizers. Used in medicine to prepare ointments, suppositories, etc., and for the manufacture of cosmetics, candles, water repellents, and polishers. In the food industry, used as a lubricant, defoamer, and raw materials for food additives such as glyceryl stearate, sorbitan stearate ester, sucrose ester, etc. For water hardness determination. In turbidimetric methods for calcium, magnesium, and lithium. Solvent for determining relative molecular weight. Active agent for acidification. Commercial stearic acid is actually a mixture of 45% stearic acid and 55% palmitic acid, and contains a small amount of oleic acid, and has a slight fatty scent. Stearic acid is widely used in cosmetics, plastic plasticizers, demolding agents, stabilizers, surfactants, rubber vulcanization promoters, water repellents, polishing agents, metal soaps, metal mineral flotation agents, softeners, pharmaceuticals, and other organic chemicals. Stearic acid can also be used as a solvent for oil-soluble pigments, crayon gliding agent, wax paper glazing agent, emulsifier for glyceryl stearate, etc. Stearic acid is widely used in the production of polyvinyl chloride pipe materials, plates, profiles, films, and is a heat stabilizer for polyvinyl chloride with good lubricity and better light and heat stabilizing effects. In polyvinyl chloride pipe materials, stearic acid helps prevent "charring" during the production process, and as a heat stabilizer in polyvinyl chloride films, it can prevent color changes in finished films due to sun exposure. Stearic acid has become a functional aid for lubrication, plasticization, and stabilization in filler modification masterbatches. Stearic acid can effectively improve the inorganic powder encapsulation and activation effect, increase material flowability. When the proportion of inorganic powders in the material is very large and requires a high melt flow rate, appropriately increasing the content of stearic acid can significantly increase the melt flow rate of the material. However, the amount of stearic acid in filler modification masterbatches also has a limit value, generally controlled at about 1% of the total mass. Adding too much can lead to a decline in the quality performance of plastic products, and may also cause paste-like substances at the die lip position of plastic product production equipment, affecting production efficiency and product quality. The monovalent or polyvalent stearic acid esters can be used as cosmetics, non-ionic surfactants, plasticizers, etc. Its alkali metal salts are soluble in water and are one of the main components of soap. Other metal salts can be used as water repellents, lubricants, fungicides, paint additives, and polyvinyl chloride stabilizers.
安全情報
安全情報を表示危険性の表示
H315
保管条件
2-8℃
感度
prone to absorb moisture
毒性
LD50 i.v. in mice, rats: 23±0.7, 21.5±1.8 mg/kg, L. Or, A. Wretlind, Acta Pharmacol. Toxicol. 18, 141 (1961)
同義語と参考文献
英語
- Stearic acid
- ACIDUM STEARICUM 50
- C18
- C18:0 FATTY ACID
- CARBOXYLIC ACID C18
- CETYLACETIC ACID
- FEMA 3035
- N-OCTADECANOIC ACID
- N-OCTADECYLIC ACID
- OCTADECANEDIOIC ACID
- RARECHEM AL BO 0157
- 1-Heptadecanecarboxylic acid
- 1-Heptadecanecarboxylic acid Ro 5-2807
- 400JB9103-88
- acideoctadecylique
- acidestearique
- AdekaFattyAcidSA910
- Barolub FTA
- Octadecanoic acid (Stearic)
- SILICA GEL HEXANE EXTRACTED MATERIAL
- Stearic Acid RG (rubber grade)
- Stearic Acid TP
- STEARIC ACID(P)
- STEARIC ACID(P) PrintBack
- yingzhisuan
- F-300, F-1000, F-1500, F-2000, F-3000
- groco54
- groco55
- groco58
- groco59
- kam1000
- naa173
- NAA180
- pd185
- Stearinic acid
- Stearophanic acid
- steraic acid
- C18:0
- NSC 25956
- NSC 261168
- Stearophanic acid
- STA
- Octadecanoic acid
- 湖北硬脂酸生产厂家行情价格
- Pearl stearic
- Stearex Beads
- Stearinsaeure
- Octadecansaeure
- Vanicol
- Century 1240
- Industrene R
- Glycon TP
- Glycon DP
- Humko Industrene R
- Formula 300
- Hydrofol 1895
- Hystrene 9718
- Hydrofol Acid 150
- Hydrofol acid 1655
- Tegostearic 254
- Hydrofol acid 1855
- Tegostearic 272
- Tegostearic 255
- Hystrene 80
- octadecoic acid
- Industrene 5016
- Emerso
- hystrene5016
- hystrene7018
- Cetylacetic acid
- 1-Heptadecanecarboxylic acid,1-Octadecanoic acid,Octadecanoic acid
- stearic acid
Beilstein_Registry_Number
608585
MDL_Number
MFCD00153444
CAS番号
57-11-4
Customs_Code
2915701000
Merck_Index
8804
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Mofu Medicine Technology Co., Ltd. 中国 - Shanghai Mofu Medicine Technology Co., Ltd. |
|||||
 中国 - Hubei Dahao Chemical Co., Ltd. 中国 - Hubei Dahao Chemical Co., Ltd. |
|||||
 中国 - Wuhan Tianzhi Biotechnology Co., Ltd. 中国 - Wuhan Tianzhi Biotechnology Co., Ltd. |
|||||
 中国 - Hengshui Zehao Rubber Chemical Co., Ltd. 中国 - Hengshui Zehao Rubber Chemical Co., Ltd. |
|||||
 中国 - Chengdu DeStock Biotechnology Co., Ltd. 中国 - Chengdu DeStock Biotechnology Co., Ltd. |
|||||
 中国 - Hubei Yunmei Technology Co., Ltd. 中国 - Hubei Yunmei Technology Co., Ltd. |
|||||
 中国 - Jiujiang Lishan Environmental Protection Technology Co., Ltd. 中国 - Jiujiang Lishan Environmental Protection Technology Co., Ltd. |
|||||
 中国 - Wenzhou Overseas Chinese Chemical Reagents Co., Ltd. 中国 - Wenzhou Overseas Chinese Chemical Reagents Co., Ltd. |
|||||
 中国 - Yixing City Yangxi Xudu Chemical Factory 中国 - Yixing City Yangxi Xudu Chemical Factory |
関連論文
Three-terminal III–V/Si tandem solar cells enabled by a transparent conductive adhesive
Manuel Schnabel, Michael Rienäcker, Emily L. Warren, Paul F. Ndione, Bill Nemeth, Talysa R. Klein, Maikel F. A. M. van Hest, John F. Geisz, Robby Peibst, Paul Stradins, Adele C. Tamboli
DOI: 10.1039/C9SE00893D
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F
Life cycle assessment of power-to-gas with biogas as the carbon source
Xiaojin Zhang, Julia Witte, Tilman Schildhauer, Christian Bauer
DOI: 10.1039/C9SE00986H
Small size yet big action: a simple sulfate anion templated a discrete 78-nuclearity silver sulfur nanocluster with a multishell structure
Li-Ping Cheng, Zhi Wang, Qiao-Yu Wu, Hai-Feng Su, Tao Peng, Geng-Geng Luo, Yan-An Li, Di Sun, Lan-Sun Zheng
DOI: 10.1039/C8CC00014J
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA delivery
Marco Herrera, Jeonghwan Kim, Yulia Eygeris, Antony Jozic
DOI: 10.1039/D0BM01947J
An elemental S/P photocatalyst for hydrogen evolution from water under visible to near-infrared light irradiation
Qiuyan Cao, Minna Guo, Jing Cao, Haili Lin, Yong Chen, Shifu Chen
DOI: 10.1039/C9CC05952K
An aminophosphonate ester ligand-containing platinum(ii) complex induces potent immunogenic cell death in vitro and elicits effective anti-tumour immune responses in vivo
Ke-Bin Huang, Feng-Yang Wang, Hai-Wen Feng, Hejiang Luo, Yan Long, Albert S. C. Chan, Rong Liu, Huahong Zou, Zhen-Feng Chen, Yan-Cheng Liu, You-Nian Liu, Hong Liang
DOI: 10.1039/C9CC06563F
Cu2ZnSnS4 nanocrystals for microwave thermal and microwave dynamic combination tumor therapy
Taya Tang, Xiaomu Xu, Zhiwen Wang, Jijing Tian, Yue Yang, Caizhang Ou, Huihui Bao, Tianlong Liu
DOI: 10.1039/C9CC07762F
Facile room-temperature growth of nanostructured CuBi2O4 for selective electrochemical reforming and photoelectrochemical hydrogen evolution reactions
Chia-Yu Lin, Shao-Yu Lin, Ming-Chun Tsai, Cheng-Hsien Wu
DOI: 10.1039/C9SE00558G

![4079-26-9 - 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole 4079-26-9 - 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole](/structs/407/4079-26-9-7725.webp)