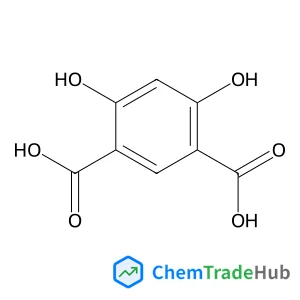
2-(1-Methylhydrazino)-4,5-dihydro-1H-imidazole hydrobromide (1:1)(CAS番号:55959-80-3)
基本情報
CAS番号
55959-80-3
分子式
C4H11BrN4
分子量
195.06 g/mol
Quick Actions
基本的な物理特性
融点
201-204°C
安全情報
安全情報を表示同義語と参考文献
英語
- N-(4,5-DIHYDROIMIDAZOL-2-YL)-N-METHYLHYDRAZINE HYDROBROMIDE
- 4-fluoro-3-(trifluoromethyl)benzylbromide
- CS-0300370
- FT-0732578
- A924109
- 2-(1-Methylhydrazino)-4,5-dihydro-1H-imidazole hydrobromide
- 2-(Methylhydrazino)-4,5-dihydro-1H-imidazole hydrobromide
- DTXSID60519981
- 2-(1-METHYLHYDRAZIN-1-YL)-4,5-DIHYDRO-1H-IMIDAZOLE HYDROBROMIDE
- AKOS024348964
- 2-(1-Methylhydrazinyl)-4,5-dihydro-1H-imidazole--hydrogen bromide (1/1)
- 1-(4,5-dihydro-1H-imidazol-2-yl)-1-methylhydrazine;hydrobromide
- 2-(1-METHYLHYDRAZINO)-2-IMIDAZOLINE HYDROBROMIDE
- 55959-80-3
- EN300-216094
- 2-(1-Methylhydrazinyl)-4,5-dihydro-1H-imidazolehydrobromide
- BP-13244
- 2-(1-Methylhydrazinyl)-4,5-dihydro-1H-imidazole hydrobromide
- 2-(1-Methylhydrazino)-2-imidazoline hydrobromide
- 1-(4,5-dihydro-1H-imidazol-2-yl)-1-methylhydrazine,hydrobromide
- 2-(1-methylhydrazin-1-yl)-4,5-dihydro-1H-imidazole hydrobromide
MDL_Number
MFCD00067859
CAS番号
55959-80-3
Customs_Code
2933990090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Hubei Qingbei Yunchen Medicine Technology Co., Ltd. 中国 - Hubei Qingbei Yunchen Medicine Technology Co., Ltd. |
|||||
 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Anhui Nanfang Chemical Pump Co., Ltd. 中国 - Anhui Nanfang Chemical Pump Co., Ltd. |
|||||
 中国 - Hebei Anping Zhongtian Chemical Factory 中国 - Hebei Anping Zhongtian Chemical Factory |
|||||
 中国 - Shanghai Jiliang Metal Materials Co., Ltd 中国 - Shanghai Jiliang Metal Materials Co., Ltd |
|||||
 ドイツ - E.Georg Lüdecke Armaturen GmbH ドイツ - E.Georg Lüdecke Armaturen GmbH |
関連論文
Boronic acid liposomes for cellular delivery and content release driven by carbohydrate binding‡
Xiaoyu Zhang, Daiane S. Alves, Jinchao Lou, Shelby D. Hill, Francisco N. Barrera, Michael D. Best
DOI: 10.1039/C8CC00820E
Enhanced power performance of an in situ sediment microbial fuel cell with steel-slag as the redox catalyst: I. electricity generation
Kyeongmin Kim, Shinya Nakashita, Tadashi Hibino
DOI: 10.1039/C9SE00918C
Stabilizing synthetic DNA for long-term data storage with earth alkaline salts
A. Xavier Kohll, Philipp L. Antkowiak, Weida D. Chen, Bichlien H. Nguyen, Wendelin J. Stark, Luis Ceze, Karin Strauss, Robert N. Grass
DOI: 10.1039/D0CC00222D
Highly efficient and durable III–V semiconductor-catalyst photocathodes via a transparent protection layer
Shinjae Hwang, James L. Young, Rachel Mow, Mengjun Li, Hongbin Yang, Philip E. Batson, Martha Greenblatt, Myles A. Steiner, Daniel Friedman, Todd G. Deutsch, Eric Garfunkel
DOI: 10.1039/C9SE01264H
Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalks
Xiaobo Zhu, Shang-wen Tang, Wenyue Zhao, Xianliang Li, Zhengyi Lv, Li Yu
DOI: 10.1039/C9SE00906J
Catalytic depolymerization of Kraft lignin to produce liquid fuels via Ni–Sn metal oxide catalysts
Baikai Zhang, Wenzhi Li, Xiaomeng Dou, Jindong Wang, Lele Jin, Ajibola T. Ogunbiyi, Xiaosen Li
DOI: 10.1039/C9SE01089K
Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloaddition
Renzeng Chen, Danbo Wang, Wenbo Hao, Feng Shao, Yingjie Zhao
DOI: 10.1039/D1CC02179F
An elemental S/P photocatalyst for hydrogen evolution from water under visible to near-infrared light irradiation
Qiuyan Cao, Minna Guo, Jing Cao, Haili Lin, Yong Chen, Shifu Chen
DOI: 10.1039/C9CC05952K
An overview of latest advances in exploring bioactive peptide hydrogels for neural tissue engineering
Pooja Sharma, Vijay Kumar Pal, Sangita Roy
DOI: 10.1039/D0BM02049D
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D


![221874-51-7 - 2-Methyl-2-propanyl [(3R)-2-oxo-3-piperidinyl]carbamate 221874-51-7 - 2-Methyl-2-propanyl [(3R)-2-oxo-3-piperidinyl]carbamate](/structs/221/221874-51-7-a692.webp)