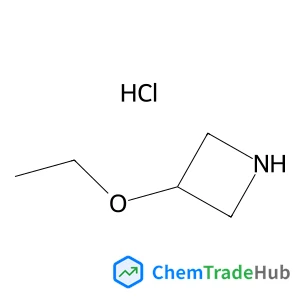
3-Ethoxyazetidine hydrochloride (1:1)(CAS番号:535924-73-3)
基本情報
CAS番号
535924-73-3
分子式
C5H12ClNO
分子量
137.61 g/mol
Quick Actions
基本的な物理特性
物理状態
Yellow to Brown Liquid
安全情報
安全情報を表示危険性の表示
H302;H315;H319;H335
保管条件
under inert gas (nitrogen or Argon) at 2-8°C
同義語と参考文献
英語
- A26673
- 3-ethoxyazetidine;hydrochloride
- SY198400
- 3-Ethoxyazetidine--hydrogen chloride (1/1)
- CS-0137902
- FZVFBLJKNJAJNI-UHFFFAOYSA-N
- 3-Ethoxyazetidine, HCl
- 3-Ethoxyazetidine hydrochloride
- DTXSID00626365
- DS-17603
- Azetidine, 3-ethoxy-, hydrochloride
- 3-Ethoxyazetidine HCl
- AB30388
- AKOS015950902
- 535924-73-3
- AM804015
- SCHEMBL5163790
- EN300-216045
- MFCD06804556
- 3-ETHOXY-AZETIDINE HYDROCHLORIDE
- 3-Ethoxyazetidinehydrochloride
- 3-Ethoxyazetidine
- 3-Ethoxyazetidine hydrochloride (1:1)
- 3-ETHOXY-AZETIDINE HYDROCHLORIDE,
- Azetidine, 3-ethoxy-, hydrochloride (1:1)
- ANW-44534
- CTK1G7636
- SureCN5163790
- WTI-10385
- AB1006624
- AB0071062
- ST2417687
- V6478
- 3-ethoxyazetidine hydrochloride
- 3-ETHOXYAZETIDINE HCL
MDL_Number
MFCD06804556
CAS番号
535924-73-3
Customs_Code
2933990090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Beijing Bailingwei Technology Co., Ltd. 中国 - Beijing Bailingwei Technology Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Shanghai Jixiang Biotechnology Co., Ltd. 中国 - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 スイス - ApiniLabs AG スイス - ApiniLabs AG |
|||||
 中国 - Shandong Mezhen Biotechnology Co., Ltd. 中国 - Shandong Mezhen Biotechnology Co., Ltd. |
|||||
 中国 - Angshan LH Chemical Industry Co., Ltd. 中国 - Angshan LH Chemical Industry Co., Ltd. |
|||||
 エストニア - OÜ TorroSen エストニア - OÜ TorroSen |
|||||
 中国 - Suzhou Sanyi Polymer Chemistry Technology Co., Ltd. 中国 - Suzhou Sanyi Polymer Chemistry Technology Co., Ltd. |
関連論文
An environmentally friendly natural polymer as a universal interfacial modifier for fullerene and non-fullerene polymer solar cells
Xiaojing Wang, Shuwang Yi, Zhicai He, Xinhua Ouyang, Hong-Bin Wu, Weiguo Zhu, Bin Zhang, Yong Cao
DOI: 10.1039/C9SE01079C
An elemental S/P photocatalyst for hydrogen evolution from water under visible to near-infrared light irradiation
Qiuyan Cao, Minna Guo, Jing Cao, Haili Lin, Yong Chen, Shifu Chen
DOI: 10.1039/C9CC05952K
Catalogue of self-targeting nano-medical inventions to accelerate clinical trials
Samar A. Alsudir
DOI: 10.1039/D1BM00235J
Near infrared light activation of an injectable whole-cell cancer vaccine for cancer immunoprophylaxis and immunotherapy
Fei Wang, Junbin Gao, Shuanghu Wang, Jiamiao Jiang, Yicheng Ye, Juanfeng Ou, Shuwen Liu, Fei Peng, Yingfeng Tu
DOI: 10.1039/D1BM00542A
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H
Heterogeneous toroidal spiral particles for islet encapsulation
Paola Leon Plata, Maryam Zaroudi, Chun-Yin Lee, Colin Foster, Ludwig C. Nitsche, Peter D. Rios, Yong Wang, Jose Oberholzer
DOI: 10.1039/D0BM02082F
Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cells
R. Vani, S. Ramaprabhu, Prathap Haridoss
DOI: 10.1039/C9SE01031A
Co9S8 integrated into nitrogen/sulfur dual-doped carbon nanofibers as an efficient oxygen bifunctional electrocatalyst for Zn–air batteries
Weiwei Zheng, Jiangquan Lv, Huabin Zhang, Hai-Xia Zhang, Jian Zhang
DOI: 10.1039/C9SE01130G
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F