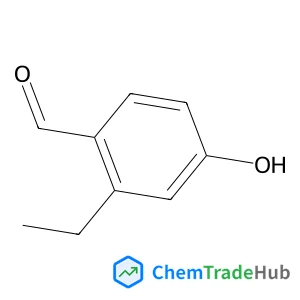
2-Ethyl-4-hydroxybenzaldehyde(CAS番号:532967-00-3)
基本情報
CAS番号
532967-00-3
分子式
C9H10O2
分子量
150.18 g/mol
Quick Actions
基本的な物理特性
融点
51-52 ºC
沸点
278.3±20.0 ºC (760 Torr),
密度
1.134±0.06 g/cm3 (20 ºC 760 Torr),
引火点
116.5±14.4 ºC,
安全情報
安全情報を表示同義語と参考文献
英語
- null
- 2-ethyl-4-hydroxybenzaldehyde
- SCHEMBL1838722
- 532967-00-3
- 2-ETHYL-4-HYDROXY-BENZALDEHYDE
- DTXSID80665358
- EN300-198932
- DA-05149
- MB23751
- NHONMDAFARLXAU-UHFFFAOYSA-N
- 2-Ethyl-4-hydroxybenzaldehyde
- Z1255421932
- Benzaldehyde, 2-ethyl-4-hydroxy- (9CI)
MDL_Number
MFCD16997641
CAS番号
532967-00-3
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 ドイツ - Romberger Maschinenfabrik GmbH ドイツ - Romberger Maschinenfabrik GmbH |
|||||
 ドイツ - Bosch Rexroth AG ドイツ - Bosch Rexroth AG |
|||||
 中国 - Guangdong Huilian_DA Chemical Co., Ltd. 中国 - Guangdong Huilian_DA Chemical Co., Ltd. |
|||||
 ドイツ - LECO Instrumente GmbH ドイツ - LECO Instrumente GmbH |
|||||
 スペイン - Tenysol S.L. スペイン - Tenysol S.L. |
|||||
 中国 - Hangzhou Rongda Pharmaceutical Chemical Co., Ltd. 中国 - Hangzhou Rongda Pharmaceutical Chemical Co., Ltd. |
関連論文
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
Palladium-catalyzed silaborative carbocyclizations of 1,6-diynes
Qian Zhang, Qiu-Ju Liang, Jian-Lin Xu, Yun-He Xu
DOI: 10.1039/C8CC00097B
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E
Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloaddition
Renzeng Chen, Danbo Wang, Wenbo Hao, Feng Shao, Yingjie Zhao
DOI: 10.1039/D1CC02179F
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosity
An-Hua Liu, Jie-Jie Li, Bai-Hao Ren, Xin-Ru Sha, He Jiang, Xiao-Bing Lu
DOI: 10.1039/C9SE00756C
Efficient one-pot synthesis of alkyl levulinate from xylose with an integrated dehydration/transfer-hydrogenation/alcoholysis process
Mengmeng Wang, Xueying Gao, Liang He, Junhua Zhang
DOI: 10.1039/C9SE00982E
Stabilizing synthetic DNA for long-term data storage with earth alkaline salts
A. Xavier Kohll, Philipp L. Antkowiak, Weida D. Chen, Bichlien H. Nguyen, Wendelin J. Stark, Luis Ceze, Karin Strauss, Robert N. Grass
DOI: 10.1039/D0CC00222D
Permselective ion electrosorption of subnanometer pores at high molar strength enables capacitive deionization of saline water
Luca Cervini
DOI: 10.1039/C9SE00996E
Effective utilisation of waste cooking oil in a single-cylinder diesel engine using alumina nanoparticles
Sumit Roy, Pranay Kumar Parsi, R. Sreeram Kotha, Sanmitra Barman, Kalluri Vinayak, Mili Mitra Roy, Rahul Banerjee
DOI: 10.1039/C9SE00393B
CaMoO4 nanosheet arrays for efficient and durable water oxidation electrocatalysis under alkaline conditions
Ying Gou, Qin Liu, Xifeng Shi, Abdullah M. Asiri, Jianming Hu, Xuping Sun
DOI: 10.1039/C8CC02092B