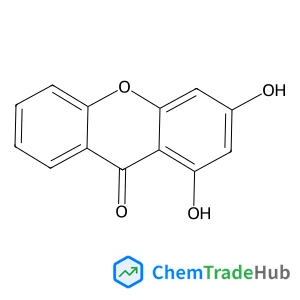
1,3-Dihydroxy-9H-xanthen-9-one(CAS番号:3875-68-1)
基本情報
CAS番号
3875-68-1
分子式
C13H8O4
分子量
228.20 g/mol
Quick Actions
基本的な物理特性
融点
259°C(lit.)
沸点
330°C (rough estimate)
引火点
199.1°C
屈折率
1.4977 (estimate)
安全情報
安全情報を表示同義語と参考文献
英語
- 9H-Xanthen-9-one,1,3-dihydroxy-
- DTXSID00192030
- 3875-68-1
- SCHEMBL2314333
- US9114126, MB1
- D5344
- 9H-Xanthen-9-one, 1,3-dihydroxy-
- 1,3-dihydroxyxanthen-9-one
- XD161541
- T70866
- CS-0181818
- 1,3-Dihydroxy-xanthone
- NS-02897
- 1,3-dihydroxy-9H-xanthen-9-one
- BDBM174832
- EN300-186368
- HMS3468P10
- 1,3-Dihydroxyxanthone
- MFCD00800670
- CHEMBL388525
- AKOS004938955
- GTHOERCJZSJGHB-UHFFFAOYSA-N
- 6,8-Dihydroxyxanthone
- 1,3-Dihydroxy-9H-xanthen-9-one
- 1,3-dihydroxy-9H-xanthone
- 1,3-dihydroxy-xanthen-9-one
- 1,3-dihydroxy-xanthene-9-one
- 1,3-dihydroxyxanthenone
- 3-dihydroxyxanthone
- AC1NUQBO
- ALBB-015550
- CTK4I0432
- SureCN2314333
- 2276AE
- STL466245
- R3833
MDL_Number
MFCD00800670
CAS番号
3875-68-1
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Leyan Reagents 中国 - Leyan Reagents |
|||||
 中国 - Shanghai Bohui Meike Chemical Technology Co., Ltd. 中国 - Shanghai Bohui Meike Chemical Technology Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Tianjin Bram Technology Co., Ltd. 中国 - Tianjin Bram Technology Co., Ltd. |
|||||
 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. |
|||||
 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 中国 - Lyn Chemical 中国 - Lyn Chemical |
関連論文
High-performance tungsten carbide electrocatalysts for the hydrogen evolution reaction
Jing Li, Bao Wang, Wei Liu
DOI: 10.1039/C9SE00853E
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Kan Zhang, Ming-Ming Yang, Shan Xu, Hua-Ming Sun, Jin-Lei Zhang, Zi-Wei Gao, Wei-Qiang Zhang
DOI: 10.1039/C8CC00324F
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosis
Shi-Yuan Zhao, Na Li, Wan-Yue Luo, Nan-Nan Zhang, Rong-Ye Zhou, Chen-Yu Li
DOI: 10.1039/D1CC90195H
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer's disease brain tissue
Ujjayini Ghosh, Wai-Ming Yau, Robert Tycko
DOI: 10.1039/C8CC01967C
An environmentally friendly natural polymer as a universal interfacial modifier for fullerene and non-fullerene polymer solar cells
Xiaojing Wang, Shuwang Yi, Zhicai He, Xinhua Ouyang, Hong-Bin Wu, Weiguo Zhu, Bin Zhang, Yong Cao
DOI: 10.1039/C9SE01079C
PEST (political, environmental, social & technical) analysis of the development of the waste-to-energy anaerobic digestion industry in China as a representative for developing countries
Habiba Khalid, Hongyan Zhang, Caiyan Liu, Wei Li, Muhammad Khubaib Abuzar, Farrukh Raza Amin, Guangqing Liu, Chang Chen
DOI: 10.1039/C9SE00692C
Developing a novel high performance NaNbO3-based lead-free dielectric capacitor for energy storage applications
DOI: 10.1039/C9SE00836E
Three-terminal III–V/Si tandem solar cells enabled by a transparent conductive adhesive
Manuel Schnabel, Michael Rienäcker, Emily L. Warren, Paul F. Ndione, Bill Nemeth, Talysa R. Klein, Maikel F. A. M. van Hest, John F. Geisz, Robby Peibst, Paul Stradins, Adele C. Tamboli
DOI: 10.1039/C9SE00893D
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J

![503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene 503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene](/structs/503/503070-57-3-bc25.webp)
![700874-71-1 - 7-[2-(4-Morpholinyl)ethoxy]-4-[2-(2-pyridinyl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline 700874-71-1 - 7-[2-(4-Morpholinyl)ethoxy]-4-[2-(2-pyridinyl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline](/structs/700/700874-71-1-fbbc.webp)









