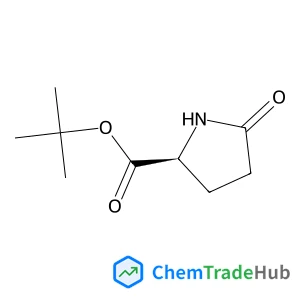
2-Methyl-2-propanyl 5-oxo-L-prolinate(CAS番号:35418-16-7)
基本情報
CAS番号
35418-16-7
分子式
C9H15NO3
分子量
185.22 g/mol
Quick Actions
基本的な物理特性
融点
102.0 to 108.0 deg-C
沸点
319.2℃ at 760 mmHg
密度
1.0990
引火点
146.8℃
屈折率
1.467
比旋光度
+6° ~ +9° (c=1, EtOH)
安全情報
安全情報を表示危険性の表示
H315,H319
同義語と参考文献
英語
- (S)-tert-Butyl 5-oxopyrrolidine-2-carboxylate
- tert-Butyl S-2-pyrrolidone-5-carboxylate
- tert-Butyl L-Pyroglutamate
- (S)-2-Pyrrolidone-5-carboxylic acid t-butyl ester
- 5-oxo-L-Proline 1,1-dimethylethyl ester
- L-Proline,5-oxo-, 1,1-dimethylethyl ester
- TERT-BUTYL (S)-2-PYRROLIDONE-5-CARBOXYLATE
- tert-butyl 5-oxo-L-prolinate
- (2S)-5-oxo-pro
- 3-broMo-9H-carbazole
- 5-Oxo-L-proline tert-butyl ester
- L-PYROGLUTAMIC ACID TERT-BUTYL ESTER
- tert-butyl (2S)-5-oxo-pyrrolidine-2-carboxylate
- tert-Butyl (S)-5-oxo-2-pyrrolidinecarboxylate
- tert-Butyl (S)-5-Oxopyrrolidine-2-carboxylate
- H-Pyr-OtBu
- (S)-5-Oxopyrrolidine-2-carboxylic Acid tert-Butyl Ester
- (S)-2-Pyrrolidone-5-carboxylic acid tert-butyl ester
- tert-Butyl 5-oxo-L-prolinate
- tert-Butyl L-pyroglutamate
- L-Pyroglutamic Acid tert-Butyl Ester
- tert-butyl (2S)-5-oxopyrrolidine-2-carboxylate
Beilstein_Registry_Number
3590226
MDL_Number
MFCD06659481
CAS番号
35418-16-7
Customs_Code
2933790090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 中国 - TICO (Shanghai) Chemical Industry Development Co., Ltd. 中国 - TICO (Shanghai) Chemical Industry Development Co., Ltd. |
|||||
 中国 - Hubei Dahao Chemical Co., Ltd. 中国 - Hubei Dahao Chemical Co., Ltd. |
|||||
 中国 - Guangdong Wanzhang Chemical Reagent Co., Ltd. 中国 - Guangdong Wanzhang Chemical Reagent Co., Ltd. |
|||||
 中国 - Tianjin Bram Technology Co., Ltd. 中国 - Tianjin Bram Technology Co., Ltd. |
|||||
 中国 - Jiangxi Besida Real Industry Co., Ltd. 中国 - Jiangxi Besida Real Industry Co., Ltd. |
|||||
 ドイツ - ZIAG Plant Engineering GmbH ドイツ - ZIAG Plant Engineering GmbH |
|||||
 中国 - Loyal Gain International Enterprise Limited 中国 - Loyal Gain International Enterprise Limited |
おすすめジャーナル

Journal of the American Chemical Society

Anti-Corrosion Methods and Materials

Journal of the Chinese Chemical Society

Cement and Concrete Research

Advances in Colloid and Interface Science

Corrosion Science

Chemical Reviews

Chemistry of Natural Compounds

AIAA Journal

Bulletin of the Chemical Society of Japan
関連論文
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosity
An-Hua Liu, Jie-Jie Li, Bai-Hao Ren, Xin-Ru Sha, He Jiang, Xiao-Bing Lu
DOI: 10.1039/C9SE00756C
Life cycle assessment of power-to-gas with biogas as the carbon source
Xiaojin Zhang, Julia Witte, Tilman Schildhauer, Christian Bauer
DOI: 10.1039/C9SE00986H
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J
Life cycle assessment of plasma-assisted ethylene production from rich-in-methane gas streams
Evangelos Delikonstantis, Elorri Igos, Michael Augustinus, Enrico Benetto
DOI: 10.1039/C9SE00736A
An improved fluorescent protein-based expression reporter system that utilizes bioluminescence resonance energy transfer and peptide-assisted complementation
Akira Takai, Keiko Yoshizawa
DOI: 10.1039/C9CC08664A
Efficient one-pot synthesis of alkyl levulinate from xylose with an integrated dehydration/transfer-hydrogenation/alcoholysis process
Mengmeng Wang, Xueying Gao, Liang He, Junhua Zhang
DOI: 10.1039/C9SE00982E
Ultra-thin NiFeSe nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting
Yu-Yang Sun, Mei-Yan Jiang, Guang-Ya Hou, Yi-Ping Tang, Min Liu
DOI: 10.1039/C9SE00905A
Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA delivery
Marco Herrera, Jeonghwan Kim, Yulia Eygeris, Antony Jozic
DOI: 10.1039/D0BM01947J
Catalogue of self-targeting nano-medical inventions to accelerate clinical trials
Samar A. Alsudir
DOI: 10.1039/D1BM00235J

![25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine 25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine](/structs/255/25553-77-9-5274.webp)