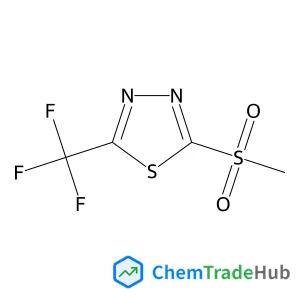
2-(Methylsulfonyl)-5-(trifluoromethyl)-1,3,4-thiadiazole(CAS番号:27603-25-4)
基本情報
CAS番号
27603-25-4
分子式
C4H3F3N2O2S2
分子量
232.21 g/mol
Quick Actions
基本的な物理特性
沸点
269 ºC
引火点
116 ºC
屈折率
1.465
安全情報
安全情報を表示危険性の表示
H302-H315-H319-H335
同義語と参考文献
英語
- AKOS016005586
- FT-0759877
- 27603-25-4
- 1,3,4-Thiadiazole, 2-(methylsulfonyl)-5-(trifluoromethyl)-
- SY128065
- AMY7246
- MFCD04092021
- 2-methylsulphonyl-5-trifluoromethyl-1,3,4-thiadiazole
- 2-(Methylsulfonyl)-5-(trifluoromethyl)-1,3,4-thiadiazole
- EC 809-978-0
- N12315
- 2-methylsulfonyl-5-(trifluoromethyl)-1,3,4-thiadiazole
- DTXSID70865385
- NS00003209
- 1,3,4-Thiadiazole,2-(methylsulfonyl)-5-(trifluoromethyl)-
- 2-methanesulfonyl-5-(trifluoromethyl)-1,3,4-thiadiazole
- SCHEMBL2207938
- A26561
- EN300-221794
- 2-?(Methylsulfonyl)?-?5-?(trifluoromethyl)?-?1,?3,?4-?thiadiazole
- DS-16796
- 2-(Methylsulfonyl)-5-(Trifluormethyl)-1,3,4-Thiadiazol
- 2-(methylsulfonyl)-5-(trifluoromethyl)-1,3,4-t
- 2-(Methylsulfonyl)-5-(Trifluoromethyl)-1,3,4-Thiadiazol
- 2-methanesulfonyl-5-trifluoromethyl-[1,3,4]-thiadiazole
- ANW-65590
- CTK8C0971
- SureCN2207938
- C4H3F3N2O2S2
- 2-(trifluoromethyl)-5-(methylsulfonyl)-1,3,4-thiadiazole
- SQLPTSMJAQPVKR-UHFFFAOYSA-N
- X5558
- 2-methanesulfonyl-5
MDL_Number
MFCD04092021
CAS番号
27603-25-4
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - HuBei Province ChuaShun Bio-Co., Ltd 中国 - HuBei Province ChuaShun Bio-Co., Ltd |
|||||
 中国 - Hubei Meiban New Materials Co., Ltd. 中国 - Hubei Meiban New Materials Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 ドイツ - ENGEMANN u. CO. int. Spedition GmbH und Co. KG ドイツ - ENGEMANN u. CO. int. Spedition GmbH und Co. KG |
|||||
 ドイツ - TeSup ドイツ - TeSup |
|||||
 イタリア - Brembana Costruzioni Industriali s.r.l. イタリア - Brembana Costruzioni Industriali s.r.l. |
|||||
 イギリス - Redd&Whyte Limited イギリス - Redd&Whyte Limited |
関連論文
An elemental S/P photocatalyst for hydrogen evolution from water under visible to near-infrared light irradiation
Qiuyan Cao, Minna Guo, Jing Cao, Haili Lin, Yong Chen, Shifu Chen
DOI: 10.1039/C9CC05952K
Engineering of electrodeposited binder-free organic-nickel hydroxide based nanohybrids for energy storage and electrocatalytic alkaline water splitting
Rohit G. Jadhav, Devraj Singh, Shaikh M. Mobin, Apurba K. Das
DOI: 10.1039/C9SE00483A
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring system
Huamin Chen, Chao Xing, Yuliang Li, Jun Wang
DOI: 10.1039/C9SE01184F
Performance of electrode-supported silica membrane separators in lithium-ion batteries
Kishen Rafiz, Y. Jin, Y. S. Lin
DOI: 10.1039/C9SE00826H
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosis
Shi-Yuan Zhao, Na Li, Wan-Yue Luo, Nan-Nan Zhang, Rong-Ye Zhou, Chen-Yu Li
DOI: 10.1039/D1CC90195H
Electrocatalytic cleavage of lignin model dimers using ruthenium supported on activated carbon cloth
Mahlet Garedew, Daniel Young-Farhat, Souful Bhatia, Pengchao Hao, James E. Jackson
DOI: 10.1039/C9SE00912D
Efficient one-pot synthesis of alkyl levulinate from xylose with an integrated dehydration/transfer-hydrogenation/alcoholysis process
Mengmeng Wang, Xueying Gao, Liang He, Junhua Zhang
DOI: 10.1039/C9SE00982E
MnO/C cubo-polyhedrons derived from α-MnO2@ZIF-8 as anode materials for high-performance lithium-ion batteries
Lei Zhang, Jiaoyu Xiao
DOI: 10.1039/C9SE00637K
Novel aqueous amine looping approach for the direct capture, conversion and storage of CO2 to produce magnesium carbonate
Meishen Liu, Hassnain Asgar, Soenke Seifert, Greeshma Gadikota
DOI: 10.1039/C9SE00316A

![503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene 503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene](/structs/503/503070-57-3-bc25.webp)









