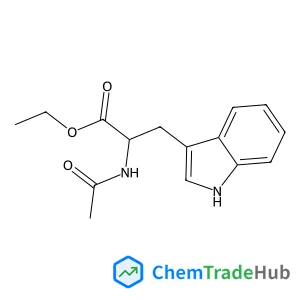
Ethyl N-acetyl-L-tryptophanate(CAS番号:2382-80-1)
基本情報
CAS番号
2382-80-1
分子式
C15H18N2O3
分子量
274.32 g/mol
Quick Actions
基本的な物理特性
融点
106.0 to 111.0 deg-C
沸点
417.3°C (rough estimate)
屈折率
6.5 ° (C=2, EtOH)
安全情報
安全情報を表示同義語と参考文献
英語
- N-ACETYLTRYPTOPHAN O-ETHYL ESTER
- J-015232
- Q27256647
- Ethyl N-acetyl-L-tryptophanate
- N-Acetyl-L-tryptophan ethyl ester (Ac-L-Trp-OEt)
- 2382-80-1
- N-Acetyl-L-tryptophan ethyl estert
- AKOS015838772
- Ac-L-Trp-OEt
- AKOS015898362
- SCHEMBL3185689
- ethyl (2S)-2-acetamido-3-(1H-indol-3-yl)propanoate
- MFCD00005643
- N.ALPHA.-ACETYL-L-TRYPTOPHAN ETHYL ESTER
- N-Acetyl-L-tryptophan ethyl ester, 99%
- Acetyl-L-tryptophan ethyl ester
- CS-W012333
- DS-16119
- Ethyl acetyl-L-tryptophanate
- EINECS 219-190-3
- N-Acetyl-L-tryptophan ethyl ester
- N_Acetyl_L_tryptophan_ethyl_ester
- A0122
- Ethyl 2-(acetylamino)-3-(1H-indol-3-yl)propanoate #
- (2S)-2-acetamido-3-(1H-indol-3-yl)propanoic acid ethyl ester
- N-ACETYLTRYPTOPHAN ETHYL ESTER
- L-Tryptophan, N-acetyl-, ethyl ester
- KQGQONPKSKUHHT-AWEZNQCLSA-N
- TRYPTOPHAN, N-ACETYL-, ETHYL ESTER, L-
- 3779MVZ8JX
- NS00050133
- A816927
- Ac-Trp-OEt
- (S)-Ethyl 2-acetamido-3-(1H-indol-3-yl)propanoate
- UNII-3779MVZ8JX
- Ethyl N-Acetyl-L-tryptophan
- N-Acetyl-L-tryptophan Ethyl Ester
- Ac-
- N-acetyl-L-tryptophane ethyl ester
- N-Acetyltryptophan ethyl ester
- N-Ac-Trp-O-Et
- Acetyl-L-Tryptophan Ethyl Ester
- ethyl (2S)-2-(acetylamino)-3-indol-3-ylpropanoate
- SBB057566
- A
MDL_Number
MFCD00005643
CAS番号
2382-80-1
Customs_Code
2933990090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Yinxin Laboratory Equipment Co., Ltd. 中国 - Shanghai Yinxin Laboratory Equipment Co., Ltd. |
|||||
 中国 - Shanghai Tengzhun BioScience Co., Ltd. 中国 - Shanghai Tengzhun BioScience Co., Ltd. |
|||||
 中国 - Shanghai Jixiang Biotechnology Co., Ltd. 中国 - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Chupeptide Biotechnology Co., Ltd. 中国 - Shanghai Chupeptide Biotechnology Co., Ltd. |
|||||
 中国 - Hubei Shixing Chemical Co., Ltd. 中国 - Hubei Shixing Chemical Co., Ltd. |
|||||
 スイス - ApiniLabs AG スイス - ApiniLabs AG |
|||||
 オーストリア - Trenka Industriebedarf Handelsgesellschaft m.b.H オーストリア - Trenka Industriebedarf Handelsgesellschaft m.b.H |
|||||
 中国 - Jiangsu Ruijia Electro-Mechanical Equipment Manufacturing Co., Ltd. 中国 - Jiangsu Ruijia Electro-Mechanical Equipment Manufacturing Co., Ltd. |
関連論文
Carbon and carbon composites obtained using deep eutectic solvents and aqueous dilutions thereof
Gaspar Carrasco-Huertas, Rafael J. Jiménez-Riobóo, María Concepción Gutiérrez, María Luisa Ferrer, Francisco del Monte
DOI: 10.1039/D0CC00681E
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring system
Huamin Chen, Chao Xing, Yuliang Li, Jun Wang
DOI: 10.1039/C9SE01184F
Transition metal chemistry in synthetically viable alkaline earth complexes M(Cp)3− (M = Ca, Sr, Ba)
Bin Huo, Rui Sun, Bo Jin, Lingfei Hu, Jian-Hong Bian, Xiao-Ling Guan, Caixia Yuan, Gang Lu, Yan-Bo Wu
DOI: 10.1039/D1CC01753E
Building microsphere–nanosheet structures in N-doped carbon to improve its performance in the oxygen reduction reaction and vanadium redox flow batteries
Baobing Huang, Yuchuan Liu, Miao Xia, Jiugen Qiu, Zailai Xie
DOI: 10.1039/C9SE00851A
Strong circularly polarized luminescence of an octahedral chromium(iii) complex
Carolin Dee, Francesco Zinna, Winald R. Kitzmann, Gennaro Pescitelli, Katja Heinze, Lorenzo Di Bari, Michael Seitz
DOI: 10.1039/C9CC06909G
Recent developments in carbon nitride based films for photoelectrochemical water splitting
Rui-Qin Zhang
DOI: 10.1039/C9SE00785G
Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer's disease brain tissue
Ujjayini Ghosh, Wai-Ming Yau, Robert Tycko
DOI: 10.1039/C8CC01967C
Electrocatalytic cleavage of lignin model dimers using ruthenium supported on activated carbon cloth
Mahlet Garedew, Daniel Young-Farhat, Souful Bhatia, Pengchao Hao, James E. Jackson
DOI: 10.1039/C9SE00912D
Pulsed laser rusted stainless steel: a robust electrode material applied for energy storage and generation applications
Namachivayam Karthik, Tian Tian, Thomas Nesakumar Jebakumar Immanuel Edison, Raji Atchudan, Yong Rok Lee, Seongbeom Kim, Dangsheng Xiong
DOI: 10.1039/C9SE00676A
Stabilizing synthetic DNA for long-term data storage with earth alkaline salts
A. Xavier Kohll, Philipp L. Antkowiak, Weida D. Chen, Bichlien H. Nguyen, Wendelin J. Stark, Luis Ceze, Karin Strauss, Robert N. Grass
DOI: 10.1039/D0CC00222D


![25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine 25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine](/structs/255/25553-77-9-5274.webp)

![700874-71-1 - 7-[2-(4-Morpholinyl)ethoxy]-4-[2-(2-pyridinyl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline 700874-71-1 - 7-[2-(4-Morpholinyl)ethoxy]-4-[2-(2-pyridinyl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline](/structs/700/700874-71-1-fbbc.webp)









