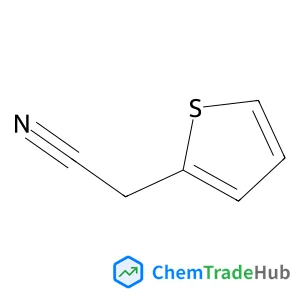
2-Thienylacetonitrile(CAS番号:20893-30-5)
2-チエニルアセトニトリル
基本情報
CAS番号
20893-30-5
分子式
C6H5NS
分子量
17.03 g/mol
Quick Actions
基本的な物理特性
融点
-5 C
沸点
120°C/22mmHg(lit.)
屈折率
n20/D 1.542(lit.)
安全情報
安全情報を表示危険性の表示
H302,H312,H315,H319,H332,H335
危険物区分
6.1
同義語と参考文献
英語
- AC-2494
- NS00007732
- AMY23238
- Thien-2-ylacetonitrile
- SCHEMBL37659
- 2-thienyl acetonitrile
- 2-thiophen-2-ylacetonitrile
- T0799
- 2-(2-thienyl)acetonitrile
- D71260
- Thiophene-2-acetonitrile
- CHEBI:27382
- 2-Thiopheneacetonitrile, 97%
- 2-Thienylacetonitrile
- EN300-21381
- F0001-0748
- 2-(Cyanomethyl)thiophene
- 2-cyanomethylthiophene
- C03311
- (Thien-2-yl)acetonitrile
- BIDD:GT0061
- PS-3265
- thiophen-2-ylacetonitrile
- Z104495654
- AKOS000119357
- AI3-08540
- FT-0638060
- W-107587
- 2-thiophene acetonitrile
- 20893-30-5
- CS-W007592
- thiophen-2-yl-acetonitrile
- 2-Thiophene-acetonitrile
- 2-Thiopheneacetonitrile
- 2-(Thiophen-2-yl)acetonitrile
- thiophen-2-acetonitrile
- MFCD00005453
- DTXSID5066663
- EINECS 244-104-6
- STR00582
- EC 244-104-6
- Q27103099
- 2-thienylacetonitrile
- 2-(2-thienyl)ethanenitrile
- 2-thiophenacetonitrile
- 2-thiophenylacetonitrile
- 2-Cyanomethylthiophene
Beilstein_Registry_Number
106960
MDL_Number
MFCD00005453
CAS番号
20893-30-5
Customs_Code
29349990
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Hubei Rike Chemical Co., Ltd. 中国 - Hubei Rike Chemical Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Wuhan HuJiu Medicine Technology Co., Ltd. 中国 - Wuhan HuJiu Medicine Technology Co., Ltd. |
|||||
 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 中国 - Jinan Aisei Medicine Technology Co., Ltd 中国 - Jinan Aisei Medicine Technology Co., Ltd |
|||||
 中国 - Hebei Jingxian Xinyuan Rubber Chemical Co., Ltd. 中国 - Hebei Jingxian Xinyuan Rubber Chemical Co., Ltd. |
|||||
 中国 - Guangzhou Xufan Trading Co., Ltd. 中国 - Guangzhou Xufan Trading Co., Ltd. |
|||||
 ドイツ - LECO Instrumente GmbH ドイツ - LECO Instrumente GmbH |
関連論文
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
Carbon and carbon composites obtained using deep eutectic solvents and aqueous dilutions thereof
Gaspar Carrasco-Huertas, Rafael J. Jiménez-Riobóo, María Concepción Gutiérrez, María Luisa Ferrer, Francisco del Monte
DOI: 10.1039/D0CC00681E
Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Sebastian Bock, Robert Zacharias, Viktor Hacker
DOI: 10.1039/C9SE00980A
MnO/C cubo-polyhedrons derived from α-MnO2@ZIF-8 as anode materials for high-performance lithium-ion batteries
Lei Zhang, Jiaoyu Xiao
DOI: 10.1039/C9SE00637K
Microscopic insights into long-range 1D ordering in a dense semi-disordered molecular overlayer
Ryan T. Hannagan, Isaac Onyango, Amanda Larson, E. Charles H. Sykes
DOI: 10.1039/D1CC01574E
An environmentally friendly natural polymer as a universal interfacial modifier for fullerene and non-fullerene polymer solar cells
Xiaojing Wang, Shuwang Yi, Zhicai He, Xinhua Ouyang, Hong-Bin Wu, Weiguo Zhu, Bin Zhang, Yong Cao
DOI: 10.1039/C9SE01079C
Engineering of electrodeposited binder-free organic-nickel hydroxide based nanohybrids for energy storage and electrocatalytic alkaline water splitting
Rohit G. Jadhav, Devraj Singh, Shaikh M. Mobin, Apurba K. Das
DOI: 10.1039/C9SE00483A
Chemoproteomics-based target profiling of sinomenine reveals multiple protein regulators of inflammation
Lianguo Chen, Hong-jian Wang, Teng-fei Ji, Chong-Jing Zhang
DOI: 10.1039/D1CC01522B
A model-based comparison of Ru and Ni catalysts for the Sabatier reaction
DOI: 10.1039/C9SE00787C
Metal–organic frameworks: preparation and applications in highly efficient heterogeneous photocatalysis
Van-Huy Nguyen, Shi-Rong Zhou, Shu-Yu Hsu, Jia-Xuan Tan
DOI: 10.1039/C9SE00972H

![697235-38-4 - Methyl (1R,2R,3S,3aR,8bS)-6-({(2S,3R,6R)-6-[(1R)-1,2-dihydroxyethyl]-3-methoxy-1,4-dioxan-2-yl}oxy)-1,8b-dihydroxy-8-methoxy-3a-(4-methoxyphenyl)-3-phenyl-2,3,3a,8b-tetrahydro-1H-benzo[b]cyclopenta[d]
furan-2-carboxylate 697235-38-4 - Methyl (1R,2R,3S,3aR,8bS)-6-({(2S,3R,6R)-6-[(1R)-1,2-dihydroxyethyl]-3-methoxy-1,4-dioxan-2-yl}oxy)-1,8b-dihydroxy-8-methoxy-3a-(4-methoxyphenyl)-3-phenyl-2,3,3a,8b-tetrahydro-1H-benzo[b]cyclopenta[d]
furan-2-carboxylate](/structs/697/697235-38-4-ee3a.webp)
![59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione) 59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione)](/structs/591/59156-70-6-0f4b.webp)
![57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone 57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone](/structs/574/57423-71-9-78dc.webp)









