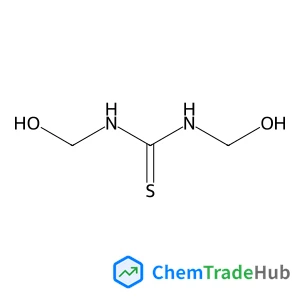
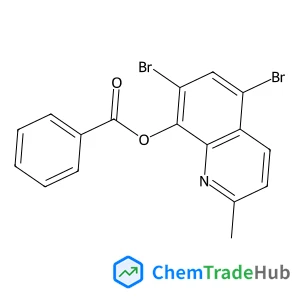
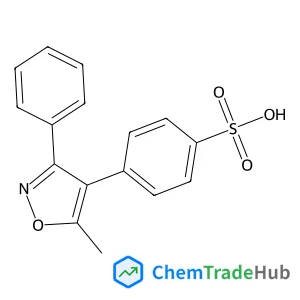
4-(5-Methyl-3-phenylisoxazol-4-yl)benzenesulfonic acid(CAS番号:181696-35-5)
基本情報
CAS番号
181696-35-5
分子式
C16H13NO4S
分子量
315.35 g/mol
Quick Actions
基本的な物理特性
安全情報
安全情報を表示危険性の表示
H302-H315-H319-H335
同義語と参考文献
英語
- 4-(5-Methyl-3-phenylisoxazol-4-yl)benzenesulfonic acid
- 2,6-DICHLORO-4-(TRIFLUOROMETHYLOXY)BROMOBEN
- VALDECOXIB IMPURITY D
- Valdecoxib Sulfonic
- Valdecoxib Sulfonic Acid
- 4-(5-Methyl-3-phenyl-1,2-oxazol-4-yl)benzenesulfonic acid
- 2,6-DICHLORO-4-(TRIFLUOROMETHYLOXY)BROMOBENZENE
- Valdecoxib IMpurity-D (Valdecoxib SulfonicAcid)
- 4-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonic Acid
- Benzenesulfonic acid, 4-(5-Methyl-3-phenyl-4-isoxazolyl)-
- 3-Phenyl-4-(4-aMinosulfonylbenzyl)-5-Methylisoxazole ,Valdecoxib Sulfonyl Chloride ,valdecoxib iMpurity D
- AK133366
- BCP23915
- BC600175
- AX8254917
- 4-(5-Methyl-3-phenyl-1,2-oxazol-4-yl)b
- 4-(5-Methyl-3-phenylisoxazol-4-yl)phenylsulfonic acid
- J-011615
- Y69WDQ5EHC
- UNII-Y69WDQ5EHC
- Valdecoxib metabolite M8
- MFCD26743607
- Benzenesulfonic acid, 4-(5-methyl-3-phenyl-4-isoxazolyl)-
- 4-(5-Methyl-3-phenylisoxazol-4-yl)phenylsulphonic acid
- C73495
- 4-(5-Methyl-3-phenylisoxazol-4-yl)benzenesulfonicacid
- Parecoxib Impurity 8; Valdecoxib Sulfonic Acid; 4-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulfonic Acid
- 4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzenesulfonic Acid
- 4-(5-Methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sulfonic acid
- AKOS016844932
- SCHEMBL6013897
- A880978
- Benzenesulphonic acid, 4-(5-methyl-3-phenyl-4-isoxazolyl)-
- ValdecoxibSulfonicAcid-13C2,15N
- DTXSID60468703
- Valdecoxib Impurity D
- DS-4699
- 4-(5-Methyl-3-phenyl-4-isoxazolyl)benzenesulphonic acid
- CS-W007260
- 181696-35-5
MDL_Number
MFCD31543896
CAS番号
181696-35-5
Customs_Code
2934999090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Zherui Biotechnology Co., Ltd. 中国 - Shanghai Zherui Biotechnology Co., Ltd. |
|||||
 中国 - Shandong Fande Medicine Technology Co., Ltd. 中国 - Shandong Fande Medicine Technology Co., Ltd. |
|||||
 中国 - Hubei Hongxin Chemical Reagents Co., Ltd. 中国 - Hubei Hongxin Chemical Reagents Co., Ltd. |
|||||
 中国 - Hubei De Ao Hua Yan Yiyao Technology Co., Ltd. 中国 - Hubei De Ao Hua Yan Yiyao Technology Co., Ltd. |
|||||
 中国 - Shanghai Kewei Chemical Technology Co., Ltd. 中国 - Shanghai Kewei Chemical Technology Co., Ltd. |
|||||
 中国 - Shenzhen Xingyue Biotechnology Co., Ltd. 中国 - Shenzhen Xingyue Biotechnology Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. |
関連論文
A robust multifunctional ligand-controlled palladium-catalyzed carbonylation reaction in water
Kan Zhang, Ming-Ming Yang, Shan Xu, Hua-Ming Sun, Jin-Lei Zhang, Zi-Wei Gao, Wei-Qiang Zhang
DOI: 10.1039/C8CC00324F
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
Catalogue of self-targeting nano-medical inventions to accelerate clinical trials
Samar A. Alsudir
DOI: 10.1039/D1BM00235J
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalysts
Minling Fang, Zhiping Zheng, Jiayu Chen, Qian Chen, Deyu Liu, Binbin Xu, Jianyang Wu, Qin Kuang
DOI: 10.1039/C9SE00678H
Effective utilisation of waste cooking oil in a single-cylinder diesel engine using alumina nanoparticles
Sumit Roy, Pranay Kumar Parsi, R. Sreeram Kotha, Sanmitra Barman, Kalluri Vinayak, Mili Mitra Roy, Rahul Banerjee
DOI: 10.1039/C9SE00393B
Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)
DOI: 10.1039/C7CC09520A
Direct arylation polycondensation towards water/alcohol-soluble conjugated polymers as the electron transporting layers for organic solar cells
Qingwu Yin, Zhenfeng Wang, Boming Xie, Fei Huang, Yong Cao
DOI: 10.1039/D1CC01128F
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F
Biomaterials Science Emerging Investigators 2021
Maria E. Southall
DOI: 10.1039/D1BM90053F

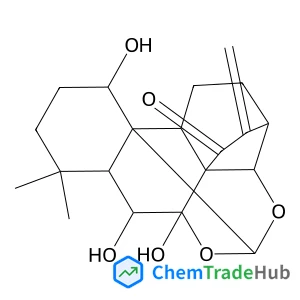
![57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone 57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone](/structs/574/57423-71-9-78dc.webp)