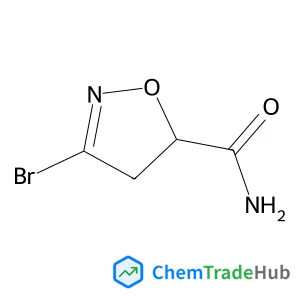
2-(2-methyl-1,3-thiazol-4-yl)phenol(CAS番号:160241-65-6)
2-(2-メチル-1,3-チオラール-4-イル)フェノール
基本情報
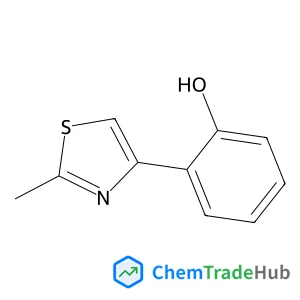
CAS番号
160241-65-6
分子式
C10H9NOS
分子量
191.26 g/mol
Quick Actions
基本的な物理特性
融点
61-64℃
沸点
331.2°Cat760mmHg
密度
1.257
引火点
154.1°C
屈折率
1.629
安全情報
安全情報を表示同義語と参考文献
英語
- EN300-110475
- 2-(2-Methyl-Thiazol-4-Yl)-Phenol
- 2-(2-Methylthiazol-4-yl)phenol
- SCHEMBL1093303
- 160241-65-6
- MFCD00665949
- AKOS002663680
- A883228
- Z1201620447
- 2-(2-METHYL-4-THIAZOLYL)PHENOL
- AS-61579
- F74380
- 2-(2-methyl-1,3-thiazol-4-yl)phenol
- Phenol, 2-(2-methyl-4-thiazolyl)-
- DTXSID70596384
- BUTTPARK 16\06-63
- 2-(2-Methyl-4-thiazolyl)phenol
- 2-(1-METHYL-1 H-TETRAZOL-5-YLSULFANYL)-ETHYLAMINE
- 2-(2-METHYL-THIAZOL-4-YL)-PHENOL
- 2,6-Dioxo-1,2,3,6-Tetrahydro-4-Pyrimidinecarbaldehyde
MDL_Number
MFCD00665949
CAS番号
160241-65-6
Customs_Code
2934100090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Hangzhou Yaorieyicheng Biopharmaceutical Co., Ltd. 中国 - Hangzhou Yaorieyicheng Biopharmaceutical Co., Ltd. |
|||||
 中国 - Shanghai Titan Shareholding Technology Co., Ltd. 中国 - Shanghai Titan Shareholding Technology Co., Ltd. |
|||||
 中国 - Hubei De Ao Hua Yan Yiyao Technology Co., Ltd. 中国 - Hubei De Ao Hua Yan Yiyao Technology Co., Ltd. |
|||||
 中国 - Shanghai Tengzhun BioScience Co., Ltd. 中国 - Shanghai Tengzhun BioScience Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. |
|||||
 中国 - Shanghai Joule Wax Industry Co., Ltd. 中国 - Shanghai Joule Wax Industry Co., Ltd. |
関連論文
Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloaddition
Renzeng Chen, Danbo Wang, Wenbo Hao, Feng Shao, Yingjie Zhao
DOI: 10.1039/D1CC02179F
The limits to biocatalysis: pushing the envelope
Roger A. Sheldon, Dean Brady
DOI: 10.1039/C8CC02463D
Life cycle assessment of power-to-gas with biogas as the carbon source
Xiaojin Zhang, Julia Witte, Tilman Schildhauer, Christian Bauer
DOI: 10.1039/C9SE00986H
Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM)
DOI: 10.1039/C7CC09520A
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring system
Huamin Chen, Chao Xing, Yuliang Li, Jun Wang
DOI: 10.1039/C9SE01184F
Palladium-catalyzed silaborative carbocyclizations of 1,6-diynes
Qian Zhang, Qiu-Ju Liang, Jian-Lin Xu, Yun-He Xu
DOI: 10.1039/C8CC00097B
In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheets
Hao Huang, Jia Li, Ya Yi, Jiahong Wang, Yihong Kang, Paul K. Chu, H. C. Ong, Xue-Feng Yu
DOI: 10.1039/C8CC00029H
A new neodymium–phosphine compound for supercapacitors with long-term cycling stability
Xiaoyu Li, Huimin Chen, Chenyu Yang, Yafeng Li
DOI: 10.1039/D1CC00650A
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G

![4079-26-9 - 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole 4079-26-9 - 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole](/structs/407/4079-26-9-7725.webp)