1-(3,5,5,6,8,8-Hexamethyl-5,6,7,8-tetrahydro-2-naphthalenyl)ethanone(CAS番号:1506-02-1)
基本情報
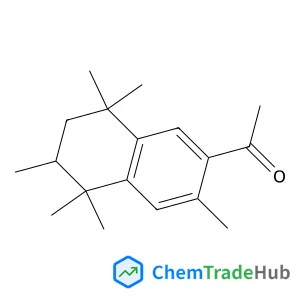
CAS番号
1506-02-1
分子式
C18H26O
分子量
258.40 g/mol
Quick Actions
基本的な物理特性
融点
52-57 °C
安全情報
安全情報を表示同義語と参考文献
英語
- 1-(1,1,2,4,4,7-hexamethyltetralin-6-yl)ethanone
- 6-Acetyl-1,1,2,4,4,7-Hexamethyltetralin
- 7-Acetyl-1,1,3,4,4,6-hexamethyltetrahydronaphthalene
- 1-(5,6,7,8-tetrahydro-3,5,5,6,8,8-hexamethyl-2-naphthalenyl)ethanone
- 5',6',7',8'-tetrahydro-3,5',5',6',8',8'-hexamethyl-2'-acetanaphthon
- 7-acetyl-1,1,3,4,4,6-hexamethyl-1,2,3,4-tetrahydronaphthalene
- AHTN
- FIXOLID
- FIXOLIDE
- tentarome
- TONALID(R) II
- TONALIDE(AHTN)
- AHTN (Tonalide)
- TONALID CRYSTALS
- ACETYL HEXAMETHYL TETRALIN
- 1-Acetyl-1,1,3,4,6-Hexamethyl
- Tonalid
- Tonalide
- 1-(3,5,5,6,8,8-hexamethyl-5,6,7,8-tetrahydronaphthalen-2-yl)ethanone
- Ethanone, 1-(5,6,7,8-tetrahydro-3,5,5,6,8,8-hexamethyl-2-naphthalenyl)-
- Tetralide
- Musk tonalid
- AHMT (perfume)
- AHMT
- acetyl methyl tetramethyl tetralin
- 7-Acetyl-1,1,3,4,4,6-hexamethyltetralin
- DNRJTBAOUJJ
MDL_Number
MFCD00437355
CAS番号
1506-02-1
Customs_Code
2914399090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Hubei Chenghai Chemical Co., Ltd. 中国 - Hubei Chenghai Chemical Co., Ltd. |
|||||
 中国 - Jiangxi Yüanshang Cōxiāo Liáojiǎn Company 中国 - Jiangxi Yüanshang Cōxiāo Liáojiǎn Company |
|||||
 中国 - Shanghai Jixiang Biotechnology Co., Ltd. 中国 - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 中国 - Jiangsu Rufen Synthetic Technology Co., Ltd. 中国 - Jiangsu Rufen Synthetic Technology Co., Ltd. |
|||||
 チリ - INQUISA S.A. チリ - INQUISA S.A. |
|||||
 ドイツ - RS Rittel & Schipper GmbH ドイツ - RS Rittel & Schipper GmbH |
|||||
 中国 - Shanxi Chenshi ZhiGuang Chemical Technology Co., Ltd. 中国 - Shanxi Chenshi ZhiGuang Chemical Technology Co., Ltd. |
|||||
 アメリカ合衆国 - AAT Bioquest, Inc. アメリカ合衆国 - AAT Bioquest, Inc. |
関連論文
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
Engineering of electrodeposited binder-free organic-nickel hydroxide based nanohybrids for energy storage and electrocatalytic alkaline water splitting
Rohit G. Jadhav, Devraj Singh, Shaikh M. Mobin, Apurba K. Das
DOI: 10.1039/C9SE00483A
Developing a novel high performance NaNbO3-based lead-free dielectric capacitor for energy storage applications
DOI: 10.1039/C9SE00836E
Three-terminal III–V/Si tandem solar cells enabled by a transparent conductive adhesive
Manuel Schnabel, Michael Rienäcker, Emily L. Warren, Paul F. Ndione, Bill Nemeth, Talysa R. Klein, Maikel F. A. M. van Hest, John F. Geisz, Robby Peibst, Paul Stradins, Adele C. Tamboli
DOI: 10.1039/C9SE00893D
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosity
An-Hua Liu, Jie-Jie Li, Bai-Hao Ren, Xin-Ru Sha, He Jiang, Xiao-Bing Lu
DOI: 10.1039/C9SE00756C
Carbon-based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels
Mufeedah Muringa Kandy
DOI: 10.1039/C9SE00827F
Direct arylation polycondensation towards water/alcohol-soluble conjugated polymers as the electron transporting layers for organic solar cells
Qingwu Yin, Zhenfeng Wang, Boming Xie, Fei Huang, Yong Cao
DOI: 10.1039/D1CC01128F
Near infrared light activation of an injectable whole-cell cancer vaccine for cancer immunoprophylaxis and immunotherapy
Fei Wang, Junbin Gao, Shuanghu Wang, Jiamiao Jiang, Yicheng Ye, Juanfeng Ou, Shuwen Liu, Fei Peng, Yingfeng Tu
DOI: 10.1039/D1BM00542A


![4079-26-9 - 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole 4079-26-9 - 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole](/structs/407/4079-26-9-7725.webp)









