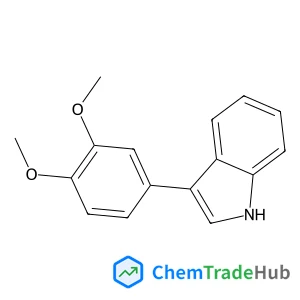
3-(3,4-Dimethoxyphenyl)-1H-indole(CAS番号:1504-15-0)
基本情報
CAS番号
1504-15-0
分子式
C16H15NO2
分子量
253.30 g/mol
Quick Actions
基本的な物理特性
沸点
435.79°C at 760 mmHg
密度
1.177
引火点
156.9°C
屈折率
1.629
安全情報
安全情報を表示同義語と参考文献
英語
- 3-(3,4-dimethoxyphenyl)-1h-indole
- 3-(3,4-Dimethoxy-phenyl)-glutarsaeure-anhydrid
- 3-<
- 3',4'-Dimethoxyphenyl>
- glutaranhydrid
- 3-(3,4-dimethoxy-phenyl)-glutaric acid-anhydride
- CTK2A7168
- 2H-Pyran-2,6(3H)-dione, dihydro-4-(3,4-dimethoxyphenyl)-
- 3-(3,4-dimethoxy-phenyl)-indole
- 3-(3,4-dimethoxyphenyl)glutaric anhydride
- 3-(m-p-OMe)-Ph-indole
- 3,4-Dimethoxy-phenyl>
- -indol
- 3-(3,4-Dimethoxy-phenyl)-indol
- 3-(3,4-dimethoxyphenyl)indole
- 3-(3,4-Dimethoxy-phenyl)-glutarsaeure-anhydrid; 3-< 3',4'-Dimethoxyphenyl> glutaranhydrid; 3-(3,4-dimethoxy-phenyl)-glutaric acid-anhydride; CTK2A7168; 2H-Pyran-2,6(3H)-dione, dihydro-4-(3,4-dimethoxyphenyl)-; 3-(3,4-dimethoxy-phenyl)-indole; 3-(3,4-dimethoxyphenyl)glutaric anhydride; 3-(m-p-OMe)-Ph-indole; 3-< 3,4-Dimethoxy-phenyl> -indol; 3-(3,4-Dimethoxy-phenyl)-indol; 3-(3,4-dimethoxyphenyl)indole;
- starbld0037191
- 1504-15-0
- AC8147
- SY251358
- SCHEMBL4329785
- JEBUUWJAEPKMST-UHFFFAOYSA-N
- 3-(3,4-Dimethoxyphenyl)-1H-indole
- MFCD17015360
MDL_Number
MFCD17015360
CAS番号
1504-15-0
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. 中国 - Jin Jinle (Hunan) Chemical Co., Ltd. |
|||||
 中国 - Shandong Yichun Chemical Co., Ltd. 中国 - Shandong Yichun Chemical Co., Ltd. |
|||||
 スイス - Embion Technologies SA スイス - Embion Technologies SA |
|||||
 ドイツ - Claudius Peters Group GmbH ドイツ - Claudius Peters Group GmbH |
|||||
 中国 - Hangzhou Hangyang Small Compressed Air Machine Co., Ltd. 中国 - Hangzhou Hangyang Small Compressed Air Machine Co., Ltd. |
|||||
 ドイツ - VRmagic GmbH ドイツ - VRmagic GmbH |
|||||
 アメリカ合衆国 - CETAC Technologies アメリカ合衆国 - CETAC Technologies |
関連論文
From Douglas fir to renewable H2-enriched syngas via ex situ catalytic pyrolysis over metal nanoparticles–nanocellulose derived carbon catalysts
Hanwu Lei, Chenxi Wang, Moriko Qian, Elmar Villota, Wendy Mateo
DOI: 10.1039/C9SE00860H
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H
Catalytic depolymerization of Kraft lignin to produce liquid fuels via Ni–Sn metal oxide catalysts
Baikai Zhang, Wenzhi Li, Xiaomeng Dou, Jindong Wang, Lele Jin, Ajibola T. Ogunbiyi, Xiaosen Li
DOI: 10.1039/C9SE01089K
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalysts
Minling Fang, Zhiping Zheng, Jiayu Chen, Qian Chen, Deyu Liu, Binbin Xu, Jianyang Wu, Qin Kuang
DOI: 10.1039/C9SE00678H
Nickel-containing N-doped carbon as effective electrocatalysts for the reduction of CO2 to CO in a continuous-flow electrolyzer
Bert De Mot, Daniel Choukroun, Chen Li, Annick Hubin, Sara Bals, Jonas Hereijgers
DOI: 10.1039/C9SE00814D
Carbon-based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels
Mufeedah Muringa Kandy
DOI: 10.1039/C9SE00827F
CaMoO4 nanosheet arrays for efficient and durable water oxidation electrocatalysis under alkaline conditions
Ying Gou, Qin Liu, Xifeng Shi, Abdullah M. Asiri, Jianming Hu, Xuping Sun
DOI: 10.1039/C8CC02092B
In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheets
Hao Huang, Jia Li, Ya Yi, Jiahong Wang, Yihong Kang, Paul K. Chu, H. C. Ong, Xue-Feng Yu
DOI: 10.1039/C8CC00029H
Stabilizing synthetic DNA for long-term data storage with earth alkaline salts
A. Xavier Kohll, Philipp L. Antkowiak, Weida D. Chen, Bichlien H. Nguyen, Wendelin J. Stark, Luis Ceze, Karin Strauss, Robert N. Grass
DOI: 10.1039/D0CC00222D


![503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene 503070-57-3 - 2-[2-(6-Bromohexyloxy)ethoxymethyl]-1,3-dichlorobenzene](/structs/503/503070-57-3-bc25.webp)
![224-53-3 - Dibenzo[c,h]acridine 224-53-3 - Dibenzo[c,h]acridine](/structs/224/224-53-3-97c9.webp)









