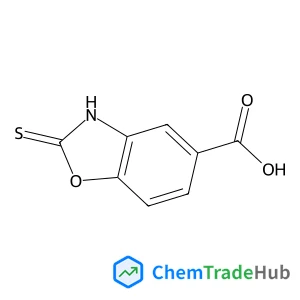
3-Fluoro-2-iodopyridine(CAS番号:146141-04-0)
基本情報
CAS番号
146141-04-0
分子式
C5H3FIN
分子量
222.99 g/mol
Quick Actions
基本的な物理特性
安全情報
安全情報を表示危険性の表示
H302
危険物区分
IRRITANT
同義語と参考文献
英語
- AMY31495
- W-205631
- AKOS015853145
- AC-27998
- DTXSID00364053
- AB13656
- 146141-04-0
- A808462
- MFCD03095288
- CS-W013107
- Pyridine,3-fluoro-2-iodo-
- 2-Iodo-3-fluoropyridine
- 3-Fluoro-2-iodo-pyridine
- 3-fluoro-2-iodopyridine
- SCHEMBL102605
- EN300-191255
- Pyridine, 3-fluoro-2-iodo-
- ZDGZTSMMMPDJLS-UHFFFAOYSA-N
- DS-20251
- SY024843
- 3-Fluoro-2-iodo-pyridine, AldrichCPR
- FT-0650497
- 3-Fluoro-2-iodopyridine
- 3-FLUORO-2-IODO-PYRIDINE
- Pyridine,3-fluoro-2-iodo
- 2-IODO-3-FLUOROPYRIDINE
- PubChem6569
- 3-fluoranyl-2-iodanyl-pyridine
- SBB096407
- AS04864
- VP11040
- TRA0042243
- RP05266
- AK689600
- AK140084
- 3-FLUORO-2-IODOPYRIDINE
MDL_Number
MFCD03095288
CAS番号
146141-04-0
Customs_Code
2933399090
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shaanxi Yudingzhuo Medicine Technology Co., Ltd. 中国 - Shaanxi Yudingzhuo Medicine Technology Co., Ltd. |
|||||
 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 中国 - Hubei Dahao Chemical Co., Ltd. 中国 - Hubei Dahao Chemical Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 ドイツ - OPTOLOGIC GmbH ドイツ - OPTOLOGIC GmbH |
|||||
 中国 - Zhengzhou Liaochuang Food Chemical Trade Co., Ltd. 中国 - Zhengzhou Liaochuang Food Chemical Trade Co., Ltd. |
|||||
 中国 - Liyang Chermlawn Chemical Co., Ltd. 中国 - Liyang Chermlawn Chemical Co., Ltd. |
|||||
 中国 - Shandong Novartis Biologics Engineering Co., Ltd. 中国 - Shandong Novartis Biologics Engineering Co., Ltd. |
おすすめジャーナル
関連論文
Engineering nanoporous organic frameworks to stabilize naked Au clusters: a charge modulation approach
Chengcheng Tian, Xiang Zhu, Huize Wang, Hai Wang, Carter W. Abney, Ning Zhang
DOI: 10.1039/C8CC02966K
Insights into the mechanism of photosynthetic H2 evolution catalyzed by a heptacoordinate cobalt complex
Fiorella Lucarini, Jennifer Fize, Adina Morozan, Mirco Natali, Mariachiara Pastore, Vincent Artero, Albert Ruggi
DOI: 10.1039/C9SE00434C
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosis
Shi-Yuan Zhao, Na Li, Wan-Yue Luo, Nan-Nan Zhang, Rong-Ye Zhou, Chen-Yu Li
DOI: 10.1039/D1CC90195H
Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cells
R. Vani, S. Ramaprabhu, Prathap Haridoss
DOI: 10.1039/C9SE01031A
Surface structure-dependent electrocatalytic reduction of CO2 to C1 products on SnO2 catalysts
Minling Fang, Zhiping Zheng, Jiayu Chen, Qian Chen, Deyu Liu, Binbin Xu, Jianyang Wu, Qin Kuang
DOI: 10.1039/C9SE00678H
Non-aqueous neptunium and plutonium redox behaviour in THF – access to a rare Np(iii) synthetic precursor
Nickolas H. Anderson, Suzanne C. Bart, Andrew J. Gaunt, Brian L. Scott
DOI: 10.1039/C8CC02611D
The limits to biocatalysis: pushing the envelope
Roger A. Sheldon, Dean Brady
DOI: 10.1039/C8CC02463D
Recent developments in carbon nitride based films for photoelectrochemical water splitting
Rui-Qin Zhang
DOI: 10.1039/C9SE00785G
Strong circularly polarized luminescence of an octahedral chromium(iii) complex
Carolin Dee, Francesco Zinna, Winald R. Kitzmann, Gennaro Pescitelli, Katja Heinze, Lorenzo Di Bari, Michael Seitz
DOI: 10.1039/C9CC06909G
Catalogue of self-targeting nano-medical inventions to accelerate clinical trials
Samar A. Alsudir
DOI: 10.1039/D1BM00235J

![500789-05-9 - (3R)-3-(2-Chlorophenyl)-3-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)propanoic acid 500789-05-9 - (3R)-3-(2-Chlorophenyl)-3-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)propanoic acid](/structs/500/500789-05-9-80b4.webp)