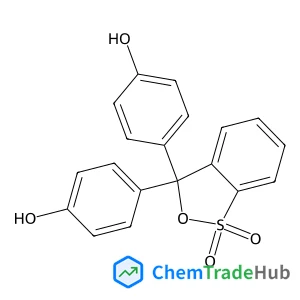
4,4'-(1,1-Dioxido-3H-2,1-benzoxathiole-3,3-diyl)diphenol(CAS番号:143-74-8)
4,4'-(1,1-ジオキソード-3H-2,1-ベンゾオキサゾリル-3,3-dioxy)dipirol Everett
基本情報
CAS番号
143-74-8
分子式
C19H14O5S
分子量
354.38 g/mol
Quick Actions
基本的な物理特性
融点
300 ºC
沸点
>300°C
水溶性
0.77 g/L
引火点
>300°C
溶解度
1 M NaOH: soluble1mg/mL
屈折率
1.5300 (estimate)
安全情報
安全情報を表示危険性の表示
H315,H319,H335
同義語と参考文献
英語
- Phenol Red
- Phenol, 4,4'-(3H-2,1-benzoxathiol-3-ylidene)bis-, S,S-dioxide
- PHENOL RED FREE ACID CELL CULTURE TESTED
- Phenol Red, indicator
- Phenolsulfonephthalein
- Phenolsulfonphthalein
- PHENOL RED PAPER, PH 6.8-PH 8.4, LENGHT: 5M
- PhenolRed,indicator,pure100GR
- PhenolRed,indicator,pure25GR
- PhenolRed,indicator,pure5GR
- 3,3-Bis(p-hydroxyphenyl)-3-H-2,1-benzoxathiol-1,1-dioxide
- 4-[3-(4-hydroxyphenyl)-1,1-dioxo-2,1λ6-benzoxathiol-3-yl]phenol
- ACS
- Phenol Red, ACS reagent
- PSP
- 3,3-Bis(p-hydroxyphenyl)-3H-2,1-benzoxathiole 1,1-dioxide
- PRGR
- NR3C3
- Phenol re
- Phenolrde
- fenolipuna
- sulphental
- sulphonthal
- Sulfonphthal
- PR
- Phenolsulphonphthalein
- PSP (indicator)
- Phenolred
- Phenol, 4,4'-(1,1-dioxido-3H-2,1-benzoxathiol-3-ylidene)bis-
- Phenosulfophthalein
- NSC10459
- 3,3-Bis(4-hydroxyphenyl)-3H-benzo[c][1,2]oxathiole 1,1-dioxide
- I6G9Y0J1OJ
- 4,4'-(3H-2,1-Benzoxathiol-3-ylidene)diphenol S,S-dioxide
- 4,4'-(1,1-
- Phenol red solution
- PHENOL RED
Beilstein_Registry_Number
0326470
MDL_Number
MFCD00003552
CAS番号
143-74-8
Customs_Code
29349990
Merck_Index
7247
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Hubei Zhenbo Chemical Industry Co., Ltd. 中国 - Hubei Zhenbo Chemical Industry Co., Ltd. |
|||||
 中国 - Wenzhou Dongsheng Chemical Reagent Factory 中国 - Wenzhou Dongsheng Chemical Reagent Factory |
|||||
 中国 - Tianjin Yunsheng Chemicals Co., Ltd. 中国 - Tianjin Yunsheng Chemicals Co., Ltd. |
|||||
 中国 - Tianjin Aoran Fine Chemical Research Institute 中国 - Tianjin Aoran Fine Chemical Research Institute |
|||||
 中国 - Chengdu TONGCHUANYUEMedicine Technology Co., Ltd 中国 - Chengdu TONGCHUANYUEMedicine Technology Co., Ltd |
|||||
 メキシコ - International Lab Supliers de México, S.A. de C.V. メキシコ - International Lab Supliers de México, S.A. de C.V. |
|||||
 中国 - Taizhou Jianjiang Tianyi Chemical Factory 中国 - Taizhou Jianjiang Tianyi Chemical Factory |
|||||
 中国 - Shanxi De Zhi Bei Metal Materials Co., Ltd. 中国 - Shanxi De Zhi Bei Metal Materials Co., Ltd. |
関連論文
An elemental S/P photocatalyst for hydrogen evolution from water under visible to near-infrared light irradiation
Qiuyan Cao, Minna Guo, Jing Cao, Haili Lin, Yong Chen, Shifu Chen
DOI: 10.1039/C9CC05952K
Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA
Matthew R. Warren, Chenzhen Zhang, Armin Vedadghavami, Krister Bokvist, Pradeep K. Dhal
DOI: 10.1039/D0BM01497D
Engineering of electrodeposited binder-free organic-nickel hydroxide based nanohybrids for energy storage and electrocatalytic alkaline water splitting
Rohit G. Jadhav, Devraj Singh, Shaikh M. Mobin, Apurba K. Das
DOI: 10.1039/C9SE00483A
Insights into the mechanism of photosynthetic H2 evolution catalyzed by a heptacoordinate cobalt complex
Fiorella Lucarini, Jennifer Fize, Adina Morozan, Mirco Natali, Mariachiara Pastore, Vincent Artero, Albert Ruggi
DOI: 10.1039/C9SE00434C
Nickel-containing N-doped carbon as effective electrocatalysts for the reduction of CO2 to CO in a continuous-flow electrolyzer
Bert De Mot, Daniel Choukroun, Chen Li, Annick Hubin, Sara Bals, Jonas Hereijgers
DOI: 10.1039/C9SE00814D
Co9S8 integrated into nitrogen/sulfur dual-doped carbon nanofibers as an efficient oxygen bifunctional electrocatalyst for Zn–air batteries
Weiwei Zheng, Jiangquan Lv, Huabin Zhang, Hai-Xia Zhang, Jian Zhang
DOI: 10.1039/C9SE01130G
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F
The dilemma between acid and base catalysis in the synthesis of benzimidazole from o-phenylenediamine and carbon dioxide‡
Martin Hulla, Simon Nussbaum, Alexy R. Bonnin, Paul J. Dyson
DOI: 10.1039/C9CC06156H

![57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone 57423-71-9 - (1R,2R,4R,6S,11R,12S,15R,18S,19R,20S,21S,23R,26R)-15-Hydroxy-11,18,21-trimethyl-5,17,24,28,29-pentaoxanonacyclo[17.9.1.1~1,20~.0~2,12~.0~4,6~.0~6,11~.0~15,19~.0~18,23~.0~21,26~]triacont-8-ene-10,16,25
,30-tetrone](/structs/574/57423-71-9-78dc.webp)