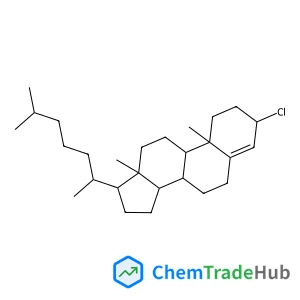
(3alpha)-3-Chlorocholest-4-ene(CAS番号:13901-10-5)
基本情報
CAS番号
13901-10-5
分子式
C27H45Cl
分子量
405.11 g/mol
Quick Actions
基本的な物理特性
融点
55-60 °C(lit.)
沸点
295.5ºC at 760mmHg
密度
1.629g/cm3
引火点
>230 °F
屈折率
1.603
安全情報
安全情報を表示同義語と参考文献
英語
- AKOS016009175
- Cholest-4-ene, 3-chloro-, (3alpha)-
- D97778
- (3R,8S,9S,10R,13R,14S,17R)-3-chloro-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,6,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene
- (3alpha)-3-chloro-Cholest-4-ene
- A886152
- 13901-10-5
- AS-60719
- 2,6-DICHLORO-3-NITROPYRIDINE
- Cholest-4-ene, 3-chloro-, (3α)-
- Cholest-4-ene,3-chloro-, (3a)-(9CI)
- TIMTEC-BB SBB003614
- -3-chloro-Cholest-4-ene
- 3-NITRO-2,6-DICHLOROPYRIDINE
- PYRIDINE, 2,6-DICHLORO-3-NITRO-
- (3Alpha)-3-chloro-Cholest-4-ene, 95+%
MDL_Number
MFCD23843785
CAS番号
13901-10-5
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 中国 - Hubei Rike Chemical Co., Ltd. 中国 - Hubei Rike Chemical Co., Ltd. |
|||||
 中国 - Jiangsu Rufen Synthetic Technology Co., Ltd. 中国 - Jiangsu Rufen Synthetic Technology Co., Ltd. |
|||||
 中国 - Hubei Shixing Chemical Co., Ltd. 中国 - Hubei Shixing Chemical Co., Ltd. |
|||||
 中国 - Shenzhen Chengfeng Intelligent Manufacturing Co., Ltd. 中国 - Shenzhen Chengfeng Intelligent Manufacturing Co., Ltd. |
|||||
 ドイツ - DURAG GmbH ドイツ - DURAG GmbH |
|||||
 中国 - Nantong Yixun Chemical Industry Co., Ltd. 中国 - Nantong Yixun Chemical Industry Co., Ltd. |
|||||
 ドイツ - Labortechnik Tasler GmbH (LTT) ドイツ - Labortechnik Tasler GmbH (LTT) |
関連論文
PEST (political, environmental, social & technical) analysis of the development of the waste-to-energy anaerobic digestion industry in China as a representative for developing countries
Habiba Khalid, Hongyan Zhang, Caiyan Liu, Wei Li, Muhammad Khubaib Abuzar, Farrukh Raza Amin, Guangqing Liu, Chang Chen
DOI: 10.1039/C9SE00692C
Visible light-driven cross-coupling reactions of alkyl halides with phenylacetylene derivatives for C(sp3)–C(sp) bond formation catalyzed by a B12 complex
Li Chen, Yohei Kametani, Kenji Imamura, Tsukasa Abe, Yoshihito Shiota, Kazunari Yoshizawa, Yoshio Hisaeda, Hisashi Shimakoshi
DOI: 10.1039/C9CC06185A
A hollow neuronal carbon skeleton with ultrahigh pyridinic N content as a self-supporting potassium-ion battery anode
Yongwen Sun, Ya Zhang, Zheng Xing, Denghu Wei, Quanchao Zhuang
DOI: 10.1039/C9SE00889F
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer's disease brain tissue
Ujjayini Ghosh, Wai-Ming Yau, Robert Tycko
DOI: 10.1039/C8CC01967C
Stabilizing synthetic DNA for long-term data storage with earth alkaline salts
A. Xavier Kohll, Philipp L. Antkowiak, Weida D. Chen, Bichlien H. Nguyen, Wendelin J. Stark, Luis Ceze, Karin Strauss, Robert N. Grass
DOI: 10.1039/D0CC00222D
Triboelectric nanogenerators for a macro-scale blue energy harvesting and self-powered marine environmental monitoring system
Huamin Chen, Chao Xing, Yuliang Li, Jun Wang
DOI: 10.1039/C9SE01184F
MnO/C cubo-polyhedrons derived from α-MnO2@ZIF-8 as anode materials for high-performance lithium-ion batteries
Lei Zhang, Jiaoyu Xiao
DOI: 10.1039/C9SE00637K
Enhanced activity of catalysts on substrates with surface protonic current in an electrical field – a review
Yudai Hisai, Quanbao Ma, Thomas Qureishy, Takeshi Watanabe, Takuma Higo, Truls Norby, Yasushi Sekine
DOI: 10.1039/D1CC01551F

![25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine 25553-77-9 - 1-[2-(1,3-Dioxolan-2-yl)ethyl]piperazine](/structs/255/25553-77-9-5274.webp)
![59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione) 59156-70-6 - 1,1'-[(1,6-Dioxo-1,6-hexanediyl)bis(oxy)]di(2,5-pyrrolidinedione)](/structs/591/59156-70-6-0f4b.webp)