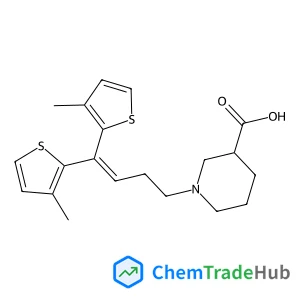
Tiagabine(CAS番号:115103-54-3)
基本情報
CAS番号
115103-54-3
分子式
C20H25NO2S2
分子量
375.56 g/mol
Quick Actions
基本的な物理特性
融点
192oC dec.
沸点
568°C at 760 mmHg
安全情報
安全情報を表示危険性の表示
H315-H319-H335
同義語と参考文献
英語
- GTPL4685
- Tiagabine [INN:BAN]
- 1-[4,4-Bis-(3-methyl-4,5-dihydro-thiophen-2-yl)-but-3-enyl]-piperidine-3-carboxylic acid
- HY-B0696
- (3R)-1-[4,4-bis(3-methylthiophen-2-yl)but-3-en-1-yl]piperidine-3-carboxylic acid
- BDBM50039251
- D08588
- DTXCID503663
- DTXSID5023663
- Tiagabinum [INN-Latin]
- Gabatril
- NCGC00164626-01
- N03AG06
- HSDB 7527
- TIAGABINE [WHO-DD]
- C07503
- Gabitril
- NO 050328
- (3R)-1-[4,4-bis(3-methylthiophen-2-yl)but-3-enyl]piperidine-3-carboxylic acid
- (R)-(-)-1-(4,4-bis(3-methyl-2-thienyl)-3-butenyl)nipecotic acid
- 1006863-69-9
- (R)-(-)-1-[4,4-bis(3-methyl-2-thienyl)-3-butenyl]nipecotic acid
- (R)-1-(4,4-bis(3-methylthiophen-2-yl)but-3-enyl)piperidine-3-carboxylic acid hydrochloride
- (R)-1-(4,4-bis(3-methylthiophen-2-yl)but-3-enyl)piperidine-3-carboxylic acid
- NO 328
- Gabitril (TN)
- 1-[4,4-Bis-(3-methyl-thiophen-2-yl)-but-3-enyl]-piperidine-3-carboxylic acid; hydrochloride
- Tiagabina
- BDBM50368628
- (3R)-1-(4,4-BIS(3-METHYL-2-THIENYL)-3-BUTEN-1-YL)-3-PIPERIDINECARBOXYLIC ACID
- AC-7048
- s4675
- NNC-050328 FREE BASE
- NO-050328 FREE BASE
- 3-Piperidinecarboxylic acid, 1-[4,4-bis(3-methyl-2-thienyl)-3-buten-1-yl]-, (3R)-
- ABBOTT-70569-1
- (-)-(R)-1-(4,4-Bis(3-methyl-2-thienyl)-3-butenyl)nipecotic acid
- TIAGABINE [MI]
- (-)-(R)-1-[4,4-Bis(3-methyl-2-thienyl)-3butenyl]nipecotic acid hydrochloride
- AMY22648
- CHEBI:9586
- Z80I64HMNP
- Tiagabine (INN)
- TIAGABINE [VANDF]
- GTPL4818
- (S)-1-(4,4-bis(3-methylthiophen-2-yl)but-3-enyl)piperidine-3-carboxylic acid
- CHEMBL1027
- NS00001014
- NO050328
- HMS3885J16
- CCG-268358
- TIAGABINE [HSDB]
- PBJUNZJWGZTSKL-MRXNPFEDSA-N
- (-)-(r)-1-[4,4-bis(3-methyl-2-thienyl)-3-butenyl]nipecotic acid
- 115103-54-3
- Q907219
- Tiagabine [INN]
- (3R)-1-[4,4-bis(3-methyl-2-thienyl)but-3-en-1-yl]piperidine-3-carboxylic acid
- (R)-1-[4,4-Bis-(3-methyl-thiophen-2-yl)-but-3-enyl]-piperidine-3-carboxylic acid; hydrochloride
- Tiagabina [INN-Spanish]
- ABBOTT-705691 FREE BASE
- NO328
- (R)-1-(4,4-Bis(3-methylthiophen-2-yl)but-3-en-1-yl)piperidine-3-carboxylic acid
- (3R)-1-[4,4-bis(3-methylthiophen-2-yl)but-3-en-1-yl]piperidine-3-carboxylic acid; Gabitril
- DB00906
- [3H]tiagabine
- (3R)-1-
- 3-Piperidinecarboxylic acid, 1-(4,4-bis(3-methyl-2-thienyl)-3-buten-1-yl)-, (3R)-
- (-)-(R)-1-
- Abbott-70569
- (R)-1-[4,4-Bis-(3-methyl-thiophen-2-yl)-but-3-enyl]-piperidine-3-carboxylic acid
- BIDD:GT0018
- Tiagabinum (INN-Latin)
- EN300-222195
- (3R)-1-(4,4-bis(3-methyl-2-thienyl)but-3-en-1-yl)piperidine-3-carboxylic acid
- ABBOTT-70569 FREE BASE
- tiagabine
- Tiagabinum
- (R)-Tiagabine
- TGB
- UNII-Z80I64HMNP
- SCHEMBL34653
- A-70569-1
- Tiagabina (INN-Spanish)
- ABT-569 FREE BASE
- 3-Piperidinecarboxylic acid, 1-(4,4-bis(3-methyl-2-thienyl)-3-butenyl)-, (R)-
- BRD-K60160658-003-08-8
- (r)-n-[4,4-bis(3-methyl-2-thienyl)but-3-en-1-yl]nipecotic acid
- AKOS016011165
- Tiagabine
- TIAGABINE, HYDROCHLORIDE
- (R)-(-)-N-(4,4-di(3-methylthien-2-yl)but-3-enyl)nipecotic acid
- (R)-1-[4,4-bis(3-methyl-2-thienyl)but-3-en-1-yl]piperidine-3-carboxylic acid
- (R)-1-[4,4-bis(3-methyl-thien-2-yl)-3-butenyl]-3-piperidinecarboxylic acid
- (R)-N-[4,4-di-(3-methylthien-2-yl)but-3-enyl] nipecotic acid hydrochloride
- 4-bis(3-methyl-2-thienyl)-3-butenyl)-1-((r)-3-piperidinecarboxylicaci
- NNC 05-328
- NO-05-0328
- no328
- TIGABINE
- (3R)-1-[4,4-Bis(3-methylthiophen-2-yl)but-3-enyl]piperidine-3-carboxylic acid
- Tiagabin
- nnc-05-0328
- NNC-05-0328:A-70569
- Gabitril / NNC 05-328
- Tiagabine 145821-59-6 /
MDL_Number
MFCD00865317
CAS番号
115103-54-3
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Shanghai Mofu Medicine Technology Co., Ltd. 中国 - Shanghai Mofu Medicine Technology Co., Ltd. |
|||||
 中国 - Hangzhou Yaorieyicheng Biopharmaceutical Co., Ltd. 中国 - Hangzhou Yaorieyicheng Biopharmaceutical Co., Ltd. |
|||||
 中国 - Wuhan Inno Pharmaceutical Technology Co., Ltd. 中国 - Wuhan Inno Pharmaceutical Technology Co., Ltd. |
|||||
 中国 - Hubei Nona Technology Co., Ltd. 中国 - Hubei Nona Technology Co., Ltd. |
|||||
 中国 - Shanghai Taifu Pharmaceutical Technology Co., Ltd. 中国 - Shanghai Taifu Pharmaceutical Technology Co., Ltd. |
|||||
 ドイツ - Zefa-Laborservice GmbH ドイツ - Zefa-Laborservice GmbH |
|||||
 ドイツ - MCI - Miritz Citrus Ingredients GmbH ドイツ - MCI - Miritz Citrus Ingredients GmbH |
|||||
 中国 - Anhui Beiyike Equipment Technology Co., Ltd. 中国 - Anhui Beiyike Equipment Technology Co., Ltd. |
関連論文
A model-based comparison of Ru and Ni catalysts for the Sabatier reaction
DOI: 10.1039/C9SE00787C
High-performance tungsten carbide electrocatalysts for the hydrogen evolution reaction
Jing Li, Bao Wang, Wei Liu
DOI: 10.1039/C9SE00853E
Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts
Xiuhui Wang, Yi Luo, Moriko Qian, Eika W. Qian
DOI: 10.1039/C9SE00682F
Pulsed laser rusted stainless steel: a robust electrode material applied for energy storage and generation applications
Namachivayam Karthik, Tian Tian, Thomas Nesakumar Jebakumar Immanuel Edison, Raji Atchudan, Yong Rok Lee, Seongbeom Kim, Dangsheng Xiong
DOI: 10.1039/C9SE00676A
Chemoproteomics-based target profiling of sinomenine reveals multiple protein regulators of inflammation
Lianguo Chen, Hong-jian Wang, Teng-fei Ji, Chong-Jing Zhang
DOI: 10.1039/D1CC01522B
Ether-functionalization of monoethanolamine (MEA) for reversible CO2 capture under solvent-free conditions with high-capacity and low-viscosity
An-Hua Liu, Jie-Jie Li, Bai-Hao Ren, Xin-Ru Sha, He Jiang, Xiao-Bing Lu
DOI: 10.1039/C9SE00756C
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosis
Shi-Yuan Zhao, Na Li, Wan-Yue Luo, Nan-Nan Zhang, Rong-Ye Zhou, Chen-Yu Li
DOI: 10.1039/D1CC90195H
Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles
Tauseef Ahmad, Sean McGrath, Catherine Sirafim, Ronaldo J. F. C. do Amaral, Shin-Loong Soong, Renuka Sitram, Shifa'a Turkistani, Francesco Santarella
DOI: 10.1039/D0BM01277G
Synthesis and optical and electronic properties of one-dimensional sulfoxonium-based hybrid metal halide (CH3)3SOPbI3
Shiqiang Bai, Xizu Wang, Si Yin Tee, Siew Lay Lim, Lin Ke, Surani B. Dolmanan, Coryl Jing Jun Lee, Poh Chong Lim, Xiang Yao, Jishan Wu
DOI: 10.1039/D1CC01386F
A new neodymium–phosphine compound for supercapacitors with long-term cycling stability
Xiaoyu Li, Huimin Chen, Chenyu Yang, Yafeng Li
DOI: 10.1039/D1CC00650A

![1337532-29-2 - 1-(5-(4-amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoroindolin-1-yl)-2-(6-methylpyridin-2-yl)ethanone 1337532-29-2 - 1-(5-(4-amino-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-4-fluoroindolin-1-yl)-2-(6-methylpyridin-2-yl)ethanone](/structs/133/1337532-29-2-edb6.webp)