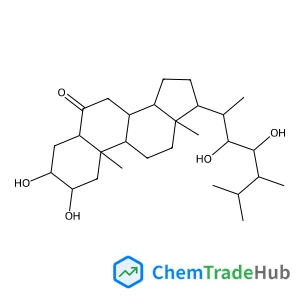
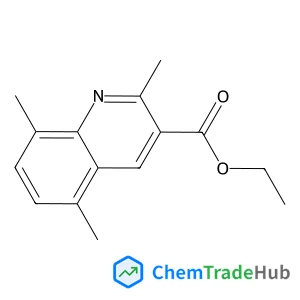
2,5,8-Trimethylquinoline-3-carboxylic acid ethyl ester(CAS番号:110139-48-5)
基本情報
CAS番号
110139-48-5
分子式
C15H17NO2
分子量
243.31 g/mol
Quick Actions
基本的な物理特性
沸点
342.9±37.0 °C at 760 mmHg
密度
1.1±0.1 g/cm3
引火点
161.2±26.5 °C
蒸気圧
0.0±0.8 mmHg at 25°C
安全情報
安全情報を表示同義語と参考文献
英語
- Ethyl 2,5,8-trimethylquinoline-3-carboxylate
- 2,5,8-Trimethylquinoline-3-carboxylic acid ethyl ester
- AGN-PC-01A9GE
- CTK8E3522
- AB51618
- 3-Quinolinecarboxylic acid, 2,5,8-trimethyl-, ethyl ester
- 2,5,8-TRIMETHYLQUINOLINE-3-CARBOXYLIC ACID ETHYL ESTER
- 110139-48-5
- DB-059978
- 2,5,8-Trimethylquinoline-3-carboxylic acid ethyl ester, AldrichCPR
- MFCD09787534
- ETHYL 2,5,8-TRIMETHYLQUINOLINE-3-CARBOXYLATE
- DTXSID80588932
- CS-0339389
MDL_Number
MFCD09787534
CAS番号
110139-48-5
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Huifei Mokai Medicinal Technology Co., Ltd. 中国 - Huifei Mokai Medicinal Technology Co., Ltd. |
|||||
 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. 中国 - Shanghai Jieshukai Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Hansi Chemical Co., Ltd. 中国 - Shanghai Hansi Chemical Co., Ltd. |
|||||
 中国 - Shanghai Jixiang Biotechnology Co., Ltd. 中国 - Shanghai Jixiang Biotechnology Co., Ltd. |
|||||
 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. 中国 - Shanghai Aladdin Bio-Technologies Co., Ltd. |
|||||
 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. 中国 - Shanghai Yuyeh Biotechnology Co., Ltd. |
|||||
 ドイツ - FVG-Filter Vertriebs GmbH ドイツ - FVG-Filter Vertriebs GmbH |
|||||
 アメリカ合衆国 - Brookhaven Instruments Corporation アメリカ合衆国 - Brookhaven Instruments Corporation |
関連論文
From Douglas fir to renewable H2-enriched syngas via ex situ catalytic pyrolysis over metal nanoparticles–nanocellulose derived carbon catalysts
Hanwu Lei, Chenxi Wang, Moriko Qian, Elmar Villota, Wendy Mateo
DOI: 10.1039/C9SE00860H
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
Building microsphere–nanosheet structures in N-doped carbon to improve its performance in the oxygen reduction reaction and vanadium redox flow batteries
Baobing Huang, Yuchuan Liu, Miao Xia, Jiugen Qiu, Zailai Xie
DOI: 10.1039/C9SE00851A
Efficient one-pot synthesis of alkyl levulinate from xylose with an integrated dehydration/transfer-hydrogenation/alcoholysis process
Mengmeng Wang, Xueying Gao, Liang He, Junhua Zhang
DOI: 10.1039/C9SE00982E
Transition-metal-free insertion reactions of alkynes into the C–N σ-bonds of imides: synthesis of substituted enamides or chromones
Zhong Zheng, Ye Wang, Murong Xu, Lingkai Kong, Mengdan Wang, Yanzhong Li
DOI: 10.1039/C8CC03059F
Performance of electrode-supported silica membrane separators in lithium-ion batteries
Kishen Rafiz, Y. Jin, Y. S. Lin
DOI: 10.1039/C9SE00826H
Retraction: Chemical synthesis and antigenic activity of a phosphatidylinositol mannoside epitope from Mycobacterium tuberculosis
Shi-Yuan Zhao, Na Li, Wan-Yue Luo, Nan-Nan Zhang, Rong-Ye Zhou, Chen-Yu Li
DOI: 10.1039/D1CC90195H
Direct arylation polycondensation towards water/alcohol-soluble conjugated polymers as the electron transporting layers for organic solar cells
Qingwu Yin, Zhenfeng Wang, Boming Xie, Fei Huang, Yong Cao
DOI: 10.1039/D1CC01128F
Synthesis and hydrogen evolving catalysis of a panchromatic photochemical molecular device
Johannes Habermehl, Djawed Nauroozi, Miłosz Martynow, Yury E. Vilk, Radim Beranek, Julien Guthmuller, Sven Rau
DOI: 10.1039/C9SE00304E
Microscopic insights into long-range 1D ordering in a dense semi-disordered molecular overlayer
Ryan T. Hannagan, Isaac Onyango, Amanda Larson, E. Charles H. Sykes
DOI: 10.1039/D1CC01574E

![4079-26-9 - 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole 4079-26-9 - 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole](/structs/407/4079-26-9-7725.webp)