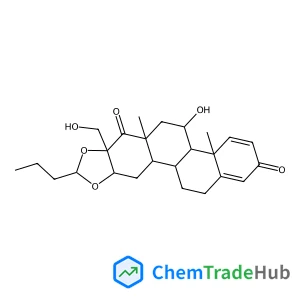
Budesonide Impurity C(CAS番号:1040085-99-1)
基本情報
CAS番号
1040085-99-1
分子式
C25H34O6
分子量
430.54 g/mol
Quick Actions
基本的な物理特性
安全情報
安全情報を表示同義語と参考文献
英語
- 16α,17-[(1RS)-Butylidenebis(oxy)]-11β-hydroxy-17-(hydroxymethyl)-D-homoandrosta-1,4-diene-3,17a-dione (Mixture of Diastereomers)
- 16α,17-[(1RS)-Butylidenebis(oxy)]-11β-hydroxy-17-(hydroxymethyl)-D-homoandrosta-1,4-diene-3,17a-dion
- 16α,17-[(1RS)-Butylidenebis(oxy)]-11β-hydroxy-17-(hydroxymethyl)-D-homoandrosta-1,4-diene-3,17a-dione (Mixture of Diastereomers)
- 16α,17-[(1RS)-Butylidenebis(oxy)]-11β-hydroxy-17-(hydroxymethyl)-D-homoandrosta-1,4-diene-3,17a-dione (Mixture of Diast...
- Budesonide IMpurity C
- Budesonide EP Impurity C
- 16α,17-[(1RS)-butylidenebis(oxy)]-11β-hydroxy-17-(hydroxymethyl)-D-homoandrosta-1,4-diene-3,17a-dione
- 16α,17-[(1RS)-Butylidenebis(oxy)]-11β-hydroxy-17-(hydroxymethyl)-D-homoandrosta-1,4-diene-3,17a-dione \n(Mixture of Diastereomers)
- (4aR,4bS,5S,6aS,7aR,10aR,11aS,11bS)-4b,6,6a,7a,10a,11,11a,11b,12,13-Decahydro-5-hydroxy-7a-(hydroxymethyl)-4a,6a-dimethyl-9-propyl-chryseno[2,3-d][1,3]dioxole-2,7(4aH,5H)-dione
- (4aR,4bS,5S,6aS,7aR,10aR,11aS,11bS)-5-hydroxy-7a-(hydroxymethyl)-4a,6a-dimethyl-9-propyl-4b,6,6a,7a,10a,11,11a,11b,12,13-decahydrochryseno[2,3-d][1,3]dioxole-2,7(4aH,5H)-dione
- Budesonide Impurity C
- Chryseno(2,3-d)(1,3)dioxole-2,7(4ah,5H)-dione, 4b,6,6a,7a,10a,11,11a,11b,12,13-decahydro-5-hydroxy-7a-(hydroxymethyl)-4a,6a-dimethyl-9-propyl-, (4aR,4bS,5S,6aS,7aR,10aR,11aS,11bS)-
- Chryseno[2,3-d][1,3]dioxole-2,7(4aH,5H)-dione, 4b,6,6a,7a,10a,11,11a,11b,12,13-decahydro-5-hydroxy-7a-(hydroxymethyl)-4a,6a-dimethyl-9-propyl-, (4aR,4bS,5S,6aS,7aR,10aR,11aS,11bS)-; 16?,17-[(1RS)-Butylidenebis(oxy)]-11?-hydroxy-17-(hydroxymethyl)-D-homo-androsta-1,4-diene-3,17a-dione; Budesonide Imp. C (EP)
- G14641
- 16alpha,17-((1RS)-Butylidenebis(oxy))-11beta-hydroxy-17-(hydroxymethyl)-D-homoandrosta-1,4-diene-3,17a-dione
- 16a,17-[(1RS)-Butylidenebis(oxy)]-11b-hydroxy-17-(hydroxymethyl)-D-homoandrosta-1,4-diene-3,17a-dione
- 16a,17-[(1RS)-Butylidenebis(oxy)]-11b-hydroxy-17-(hydroxymethyl)-D-homoandrosta-1,4-diene-3,17a-dione (Mixture of Diastereomers)
- 7BYQ3VT7JN
- 16alpha,17-[(1RS)-Butylidenebis(oxy)]-11beta-hydroxy-17-(hydroxymethyl)-D-homoandrosta-1,4-diene-3,17a-dion
- (4aR,4bS,5S,6aS,7aR,10aR,11aS,11bS)-5-hydroxy-7a-(hydroxymethyl)-4a,6a-dimethyl-9-propyl-5,6,10a,11,11a,11b,12,13-octahydro-4bH-chryseno[2,3-d][1,3]dioxole-2,7-dione
- D-HOMOBUDESONIDE
- 16?,17-[(1RS)-Butylidenebis(oxy)]-11?-hydroxy-17-(hydroxymethyl)-D-homo-androsta-1,4-diene-3,17a-dione
- Budesonide impurity C [EP]
- 16.ALPHA.,17-((1RS)-BUTYLIDENEBIS(OXY))-11.BETA.-HYDROXY-17-(HYDROXYMETHYL)-D-HOMOANDROSTA-1,4-DIENE-3,17A-DIONE
- Chryseno[2,3-d][1,3]dioxole-2,7(4aH,5H)-dione, 4b,6,6a,7a,10a,11,11a,11b,12,13-decahydro-5-hydroxy-7a-(hydroxymethyl)-4a,6a-dimethyl-9-propyl-, (4aR,4bS,5S,6aS,7aR,10aR,11aS,11bS)-
- UNII-7BYQ3VT7JN
- BUDESONIDE IMPURITY C [EP IMPURITY]
- 1040085-99-1
MDL_Number
MFCD29920082
CAS番号
1040085-99-1
サプライヤー情報
| サプライヤー名 | 会員レベル | 認証状況 | 主要カテゴリー | 最小注文数量 | アクション |
|---|---|---|---|---|---|
 中国 - Famous Biology & Technology Co., Ltd., Hubei Province 中国 - Famous Biology & Technology Co., Ltd., Hubei Province |
|||||
 中国 - Hubei Yangxin Pharmaceutical Technology Co., Ltd. 中国 - Hubei Yangxin Pharmaceutical Technology Co., Ltd. |
|||||
 中国 - Hubei De Ao Hua Yan Yiyao Technology Co., Ltd. 中国 - Hubei De Ao Hua Yan Yiyao Technology Co., Ltd. |
|||||
 中国 - Shanghai Kewei Chemical Technology Co., Ltd. 中国 - Shanghai Kewei Chemical Technology Co., Ltd. |
|||||
 中国 - Shijiazhuang Yaobo Medicine Technology Co., Ltd. 中国 - Shijiazhuang Yaobo Medicine Technology Co., Ltd. |
|||||
 中国 - Shanghai Yuansi Biotechnology Co., Ltd. 中国 - Shanghai Yuansi Biotechnology Co., Ltd. |
|||||
 中国 - Hubei Rieno Pharmaceutical Technology Co., Ltd. 中国 - Hubei Rieno Pharmaceutical Technology Co., Ltd. |
|||||
 中国 - Jiangmen Hengyi Real Industry Co., Ltd. 中国 - Jiangmen Hengyi Real Industry Co., Ltd. |
関連論文
Tessellation strategy for the interfacial synthesis of an anthracene-based 2D polymer via [4+4]-photocycloaddition
Renzeng Chen, Danbo Wang, Wenbo Hao, Feng Shao, Yingjie Zhao
DOI: 10.1039/D1CC02179F
Transition metal chemistry in synthetically viable alkaline earth complexes M(Cp)3− (M = Ca, Sr, Ba)
Bin Huo, Rui Sun, Bo Jin, Lingfei Hu, Jian-Hong Bian, Xiao-Ling Guan, Caixia Yuan, Gang Lu, Yan-Bo Wu
DOI: 10.1039/D1CC01753E
Life cycle assessment of power-to-gas with biogas as the carbon source
Xiaojin Zhang, Julia Witte, Tilman Schildhauer, Christian Bauer
DOI: 10.1039/C9SE00986H
Carbon-based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels
Mufeedah Muringa Kandy
DOI: 10.1039/C9SE00827F
Cu2ZnSnS4 nanocrystals for microwave thermal and microwave dynamic combination tumor therapy
Taya Tang, Xiaomu Xu, Zhiwen Wang, Jijing Tian, Yue Yang, Caizhang Ou, Huihui Bao, Tianlong Liu
DOI: 10.1039/C9CC07762F
Catalogue of self-targeting nano-medical inventions to accelerate clinical trials
Samar A. Alsudir
DOI: 10.1039/D1BM00235J
In situ growth of all-inorganic perovskite nanocrystals on black phosphorus nanosheets
Hao Huang, Jia Li, Ya Yi, Jiahong Wang, Yihong Kang, Paul K. Chu, H. C. Ong, Xue-Feng Yu
DOI: 10.1039/C8CC00029H
Mechanism of lignocellulose modification and enzyme disadsorption for complete biomass saccharification to maximize bioethanol yield in rapeseed stalks
Xiaobo Zhu, Shang-wen Tang, Wenyue Zhao, Xianliang Li, Zhengyi Lv, Li Yu
DOI: 10.1039/C9SE00906J
Selective production of monocyclic aromatic hydrocarbons from ex situ catalytic fast pyrolysis of pine over the HZSM-5 catalyst with calcium formate as a hydrogen source
Kai Li, Ze-xiang Wang, Guan Zhang, Min-shu Cui, Qiang Lu, Yong-ping Yang
DOI: 10.1039/C9SE00605B
Biomimetic hydrogels designed for cartilage tissue engineering
Alexander Stokes, Piergiorgio Gentile, Ana M. Ferreira
DOI: 10.1039/D0BM01852J

![500789-05-9 - (3R)-3-(2-Chlorophenyl)-3-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)propanoic acid 500789-05-9 - (3R)-3-(2-Chlorophenyl)-3-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)propanoic acid](/structs/500/500789-05-9-80b4.webp)